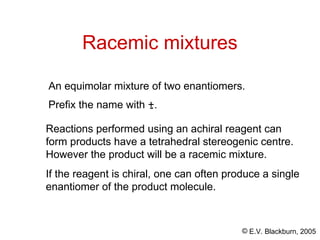
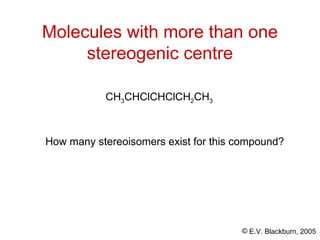
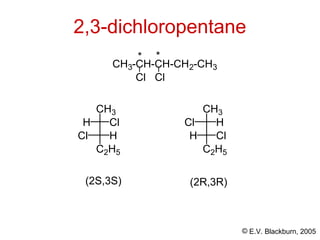
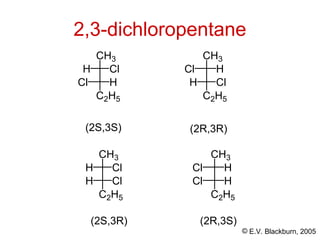
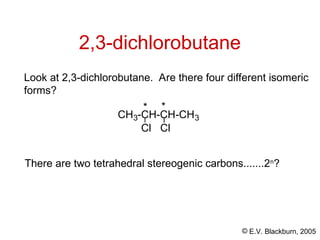
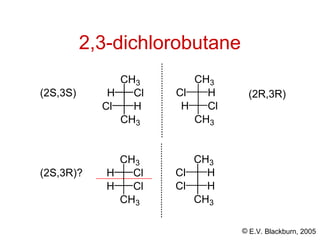
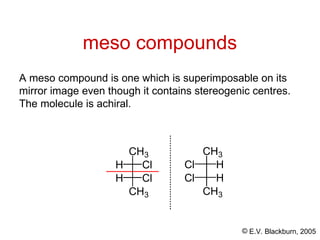
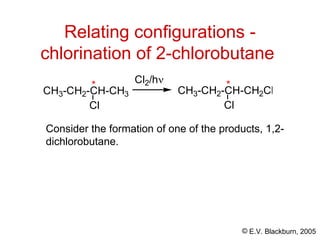
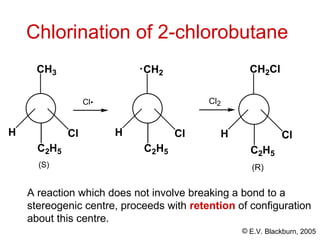
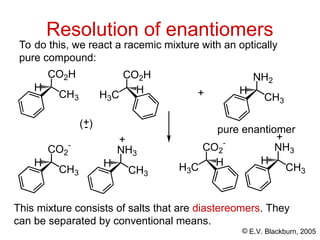
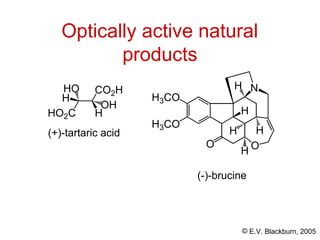
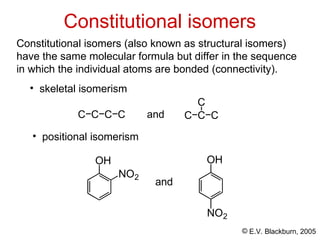
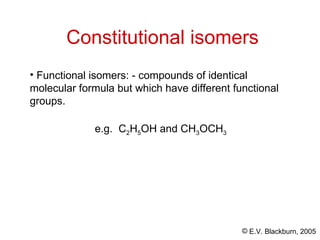
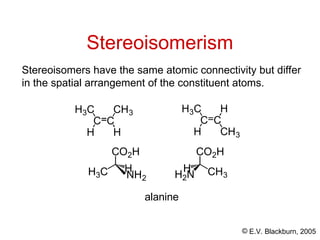
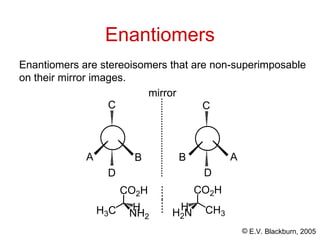
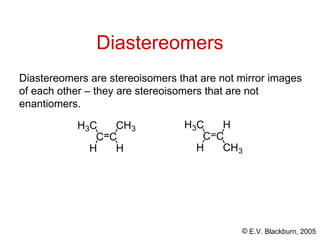
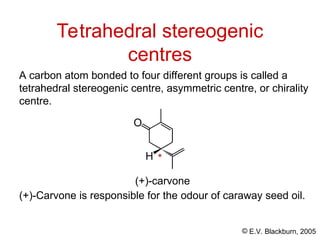
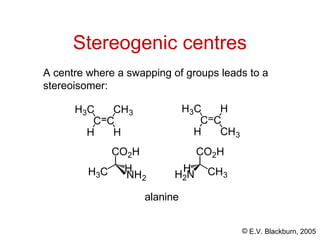
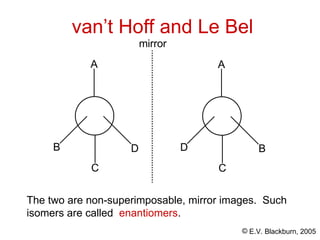
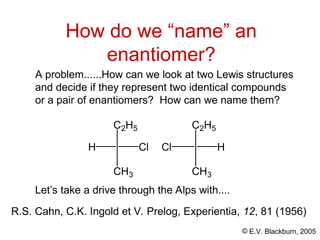
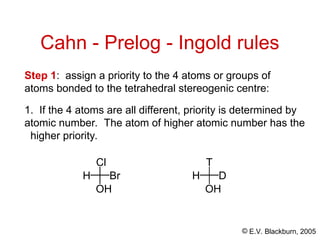
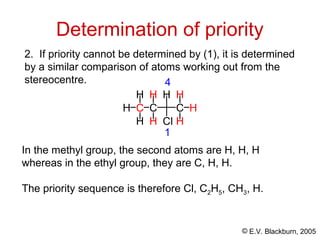
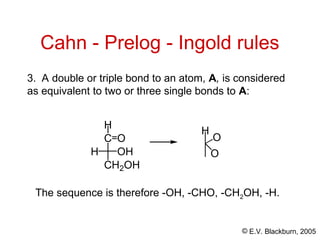
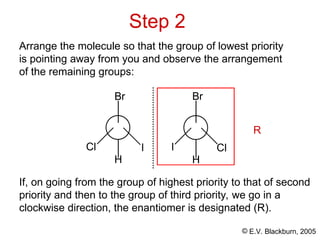
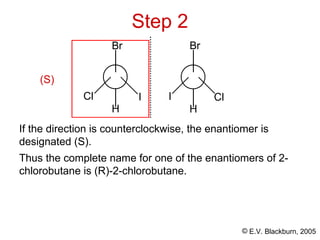
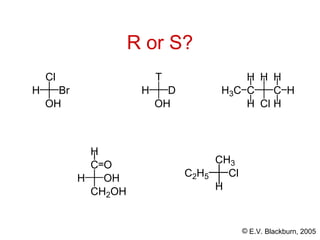
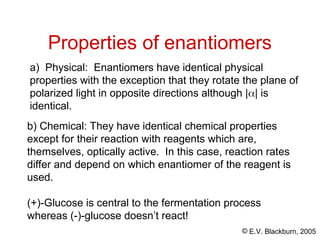
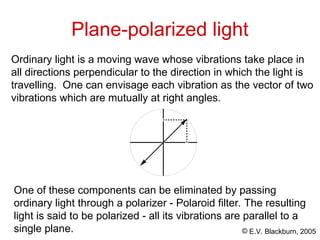
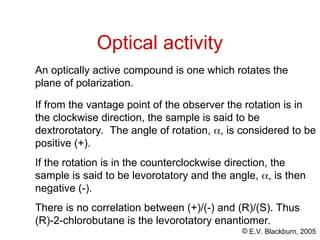
The document provides an overview of stereochemistry, detailing the three major types of isomerism: constitutional, geometrical, and optical isomerism. It describes stereoisomers and their classifications, including enantiomers and diastereomers, along with chirality and tetrahedral stereogenic centers. Additionally, it discusses the properties of enantiomers, optical activity, methods for naming them, and how to resolve racemic mixtures into individual enantiomers.




![© E.V. Blackburn, 2005
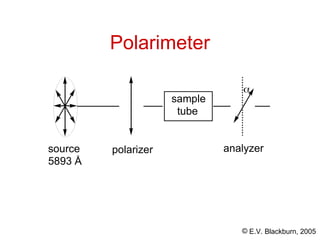
Specific rotation
is proportional to the concentration of the sample
and the length of the sample tube:
[]
t
=
l x c
- angle of rotation measured in degrees
t - temperature
- wavelength of light
l - length of sample cell
c - concentration in grams of substance contained
in 1 mL of solution](https://image.slidesharecdn.com/stereochemistry-240825204752-fce1c0fc/85/STEREOCHEMISTRY-for-basic-learning-by-students-29-320.jpg)