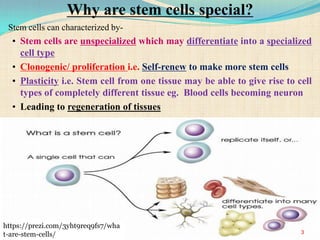
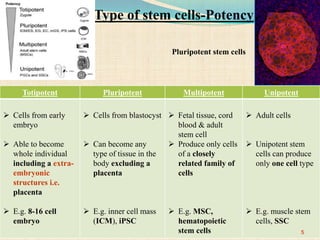
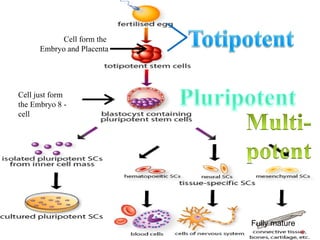
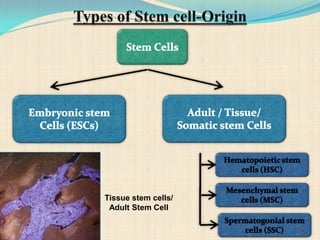
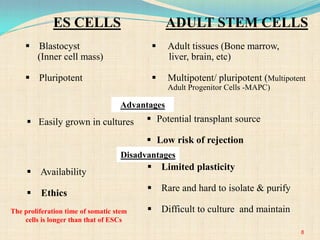
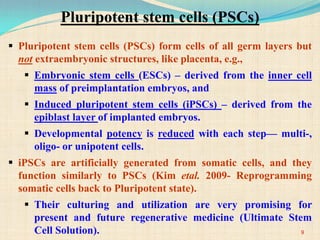
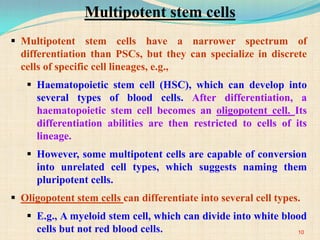
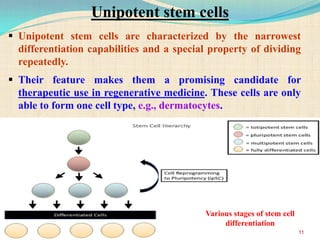
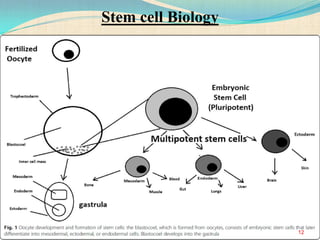
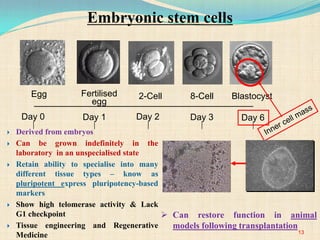
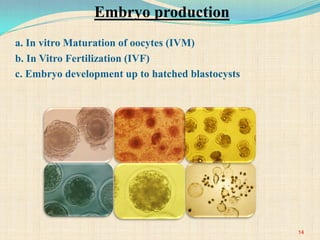
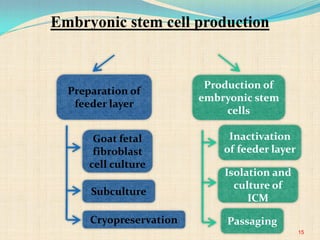
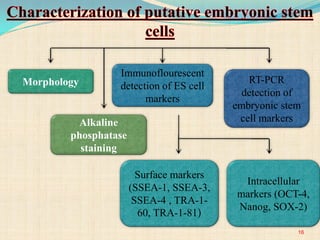
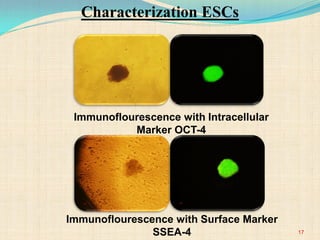
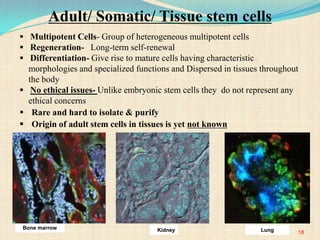
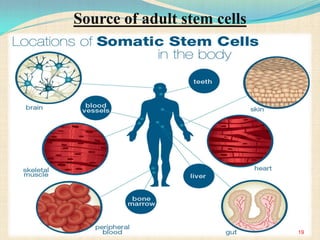
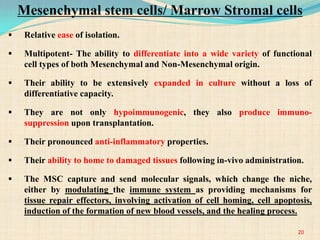
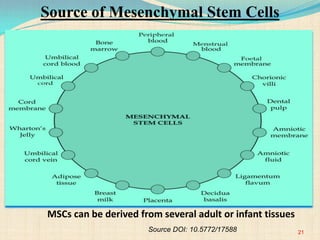
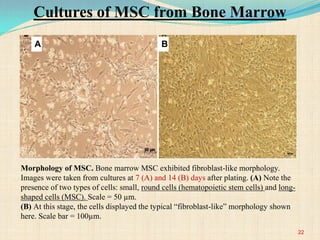
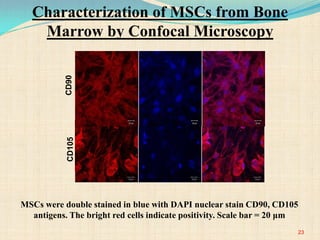
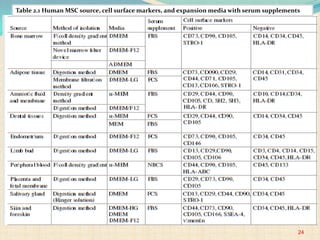
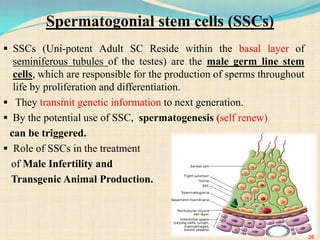
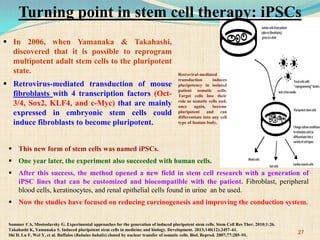
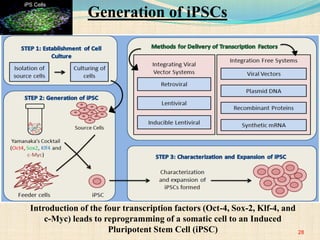
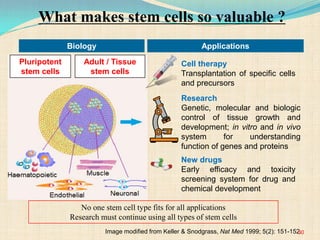
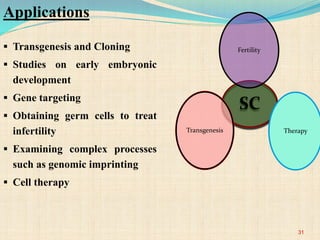
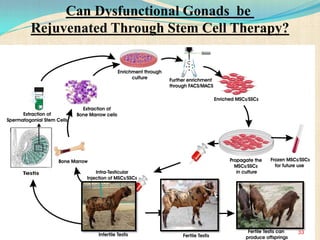
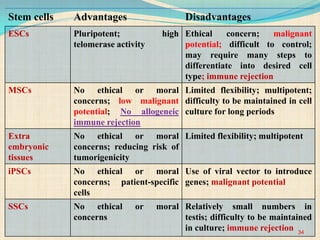
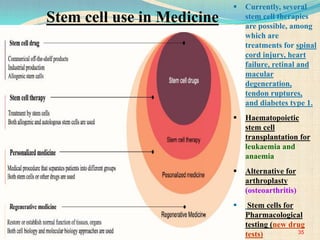
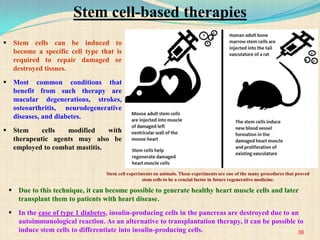
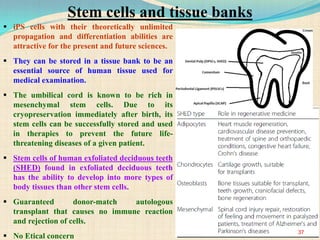
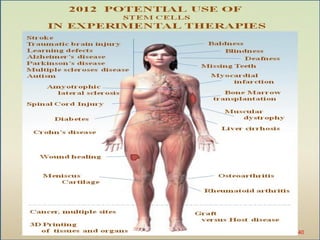
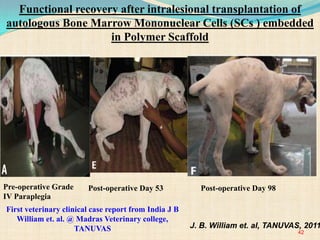
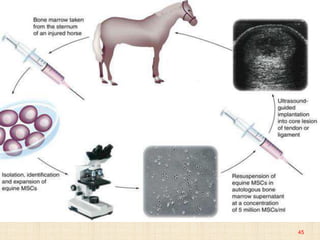
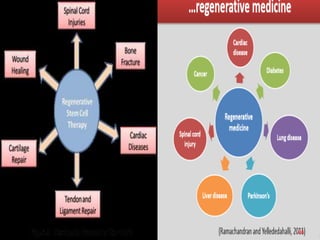
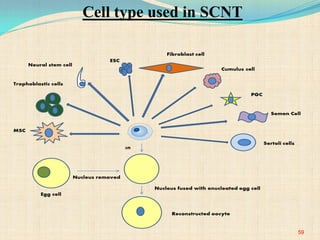
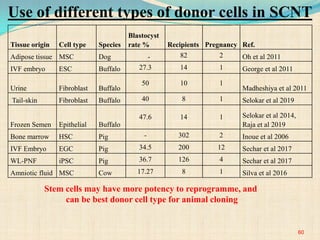
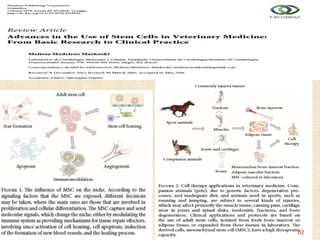
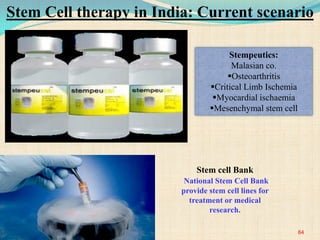
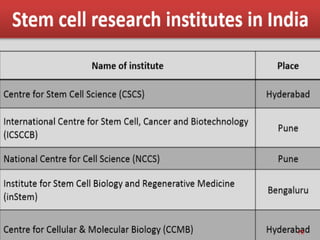
The document discusses stem cells, including their characteristics, differentiation potential, historical milestones, and various types such as embryonic, induced pluripotent, and mesenchymal stem cells. It highlights their applications in regenerative medicine, particularly in treating conditions like diabetes, heart disease, and other degenerative disorders. Additionally, it addresses ethical concerns, advantages, and disadvantages associated with different stem cell types and therapies.