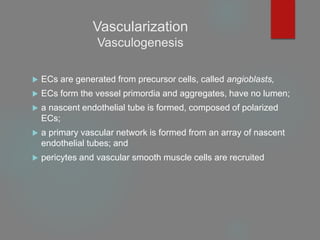
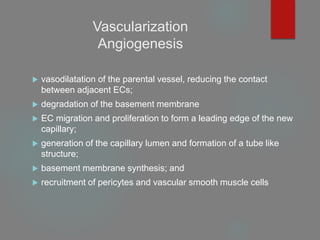
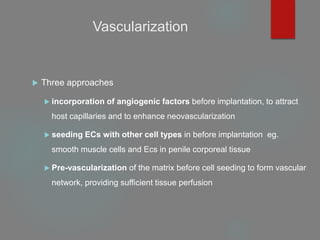
This document provides an overview of principles of tissue engineering. It discusses why tissue engineering is needed due to limited organ transplantation availability. Tissue engineering uses regenerative medicine approaches including cell therapies, biomaterials, and tissue engineering to repair or replace damaged tissues. Various cell sources for therapy are described, including stem cells (embryonic, adult, perinatal), somatic cell nuclear transfer, and induced pluripotent stem cells. Biomaterials are discussed that can be used as scaffolds to support cell growth. The importance of vascularization for tissue volumes over 3mm is also highlighted.






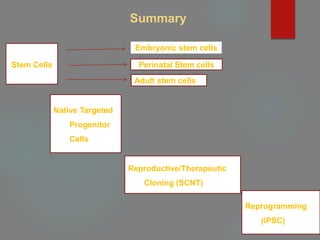
![A] Stem Cells
Defined as having three important properties:
Ability to self-renew,
Ability to differentiate into a number of different cell types,
Ability to easily form clonal populations
Autologous, Allogenic](https://image.slidesharecdn.com/1principlesoftissueengineering-200716071253/85/1-principles-of-tissue-engineering-7-320.jpg)

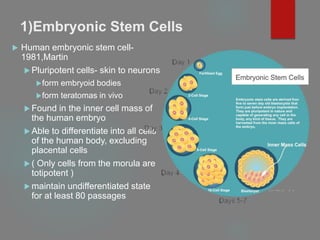


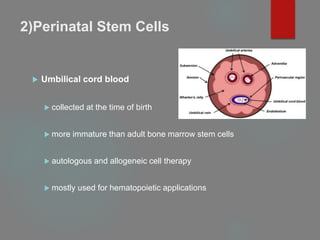
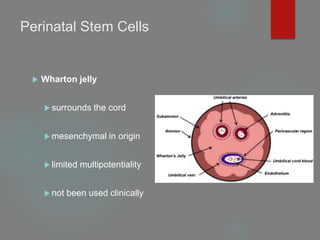


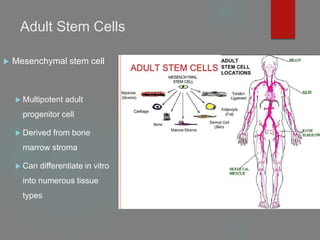

![B] Native Targeted Progenitor Cells
Tissue specific unipotent cells derived from most organ
Already programmed to become the cell type needed, without
any extra-lineage differentiation
obtained from the specific organ to be regenerated, expanded,
and used in the same patient without rejection](https://image.slidesharecdn.com/1principlesoftissueengineering-200716071253/85/1-principles-of-tissue-engineering-18-320.jpg)



![C] Somatic Cell Nuclear Transfer
Removal of an oocyte nucleus in culture, followed by its
replacement with a nucleus derived from a somatic cell obtained
from a patient
Two types of cloning
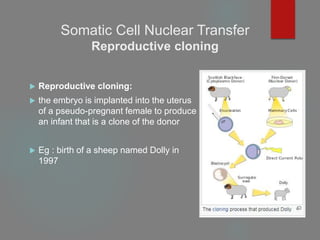
Reproductive cloning
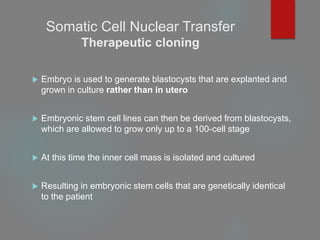
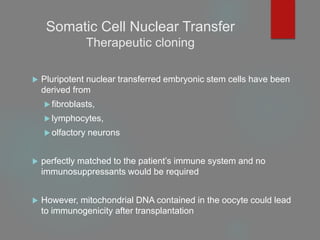
Therapeutic cloning
Both involve the insertion of donor DNA into an enucleated
oocyte to generate an embryo that has identical genetic material
to its DNA source.](https://image.slidesharecdn.com/1principlesoftissueengineering-200716071253/85/1-principles-of-tissue-engineering-22-320.jpg)

![D] Reprogramming
(Induced Pluripotent Stem Cells)
Involves de-differentiation of adult somatic cells to produce
patient-specific pluripotent stem cells, eliminating the need to
create embryos
genetically identical to the somatic cells and would not be
rejected
iPS cells possessed the characteristics of
self-renewing embryonic stem cells
expressed genes specific for embryonic stem cells
generated embryoid bodies in vitro
teratomas in vivo](https://image.slidesharecdn.com/1principlesoftissueengineering-200716071253/85/1-principles-of-tissue-engineering-27-320.jpg)