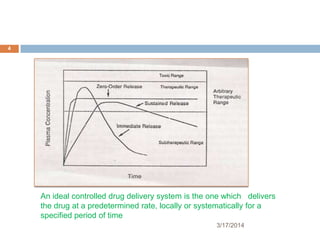
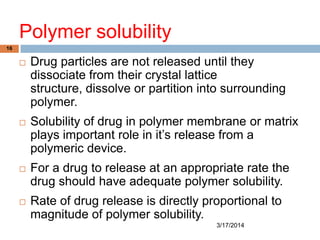
This document discusses the effects of various system parameters on controlled drug delivery systems. It describes parameters like polymer solubility, solution solubility, polymer and solution diffusivity, thickness of the polymer and hydrodynamic diffusion layers, partition coefficient, drug loading dose, and surface area. These parameters influence the rate and duration of drug release from controlled release formulations.