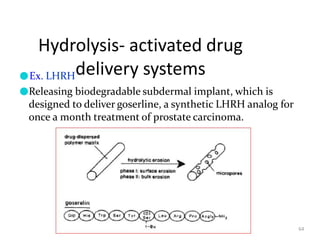
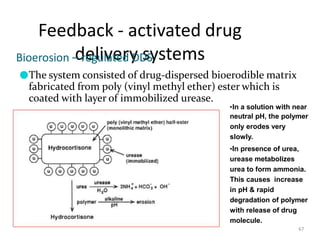
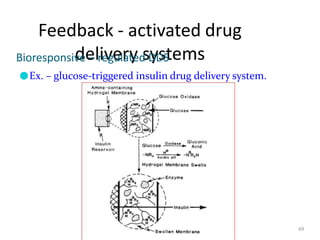
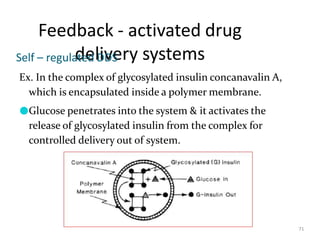
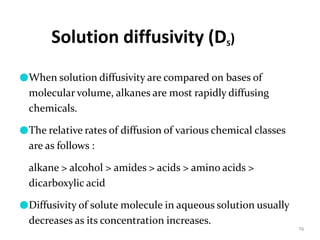
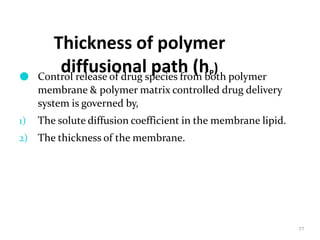
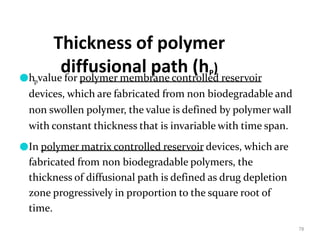
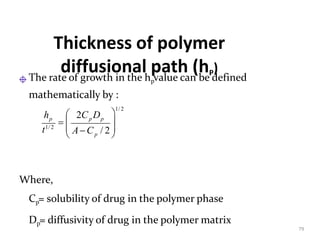
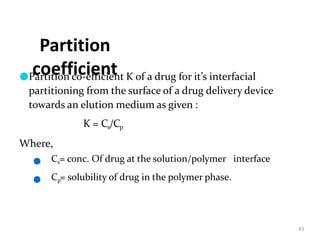
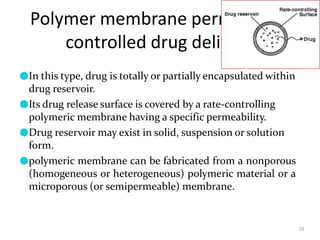
1. The document discusses different types of rate controlled drug delivery systems including preprogrammed and activation modulated systems.
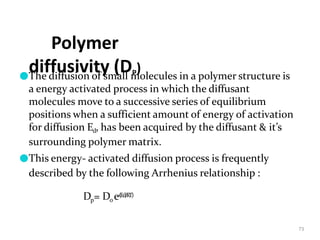
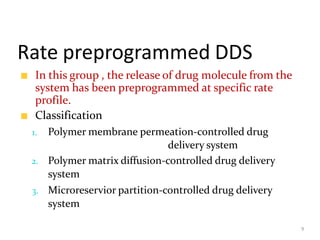
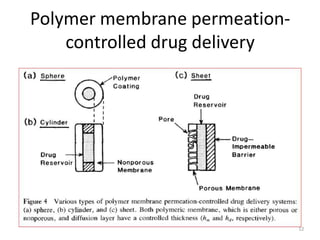
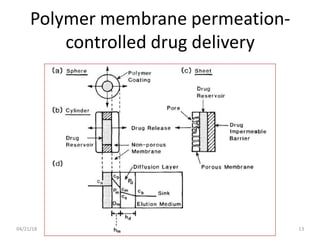
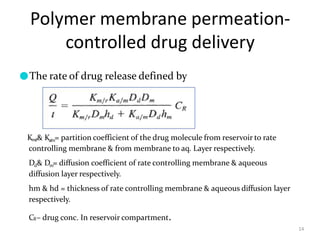
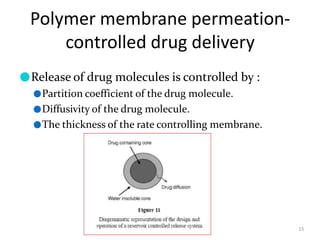
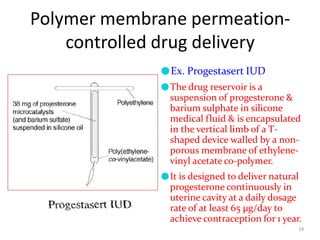
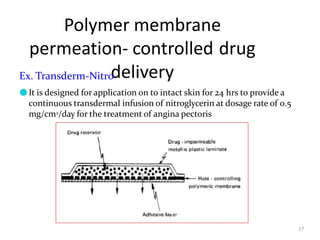
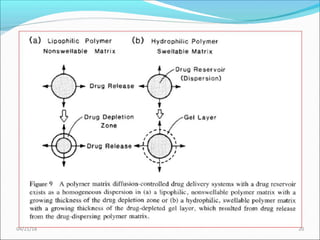
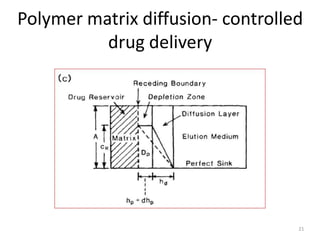
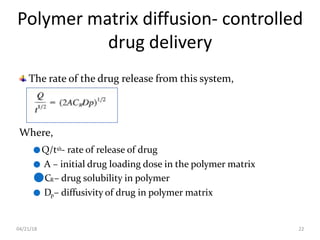
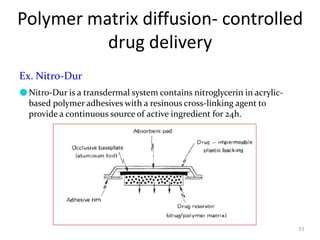
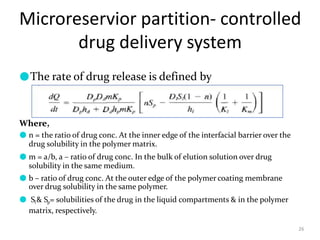
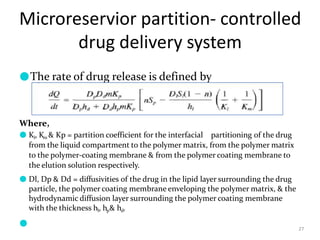
2. Preprogrammed systems include polymer membrane permeation controlled, polymer matrix diffusion controlled, and microreservoir partition controlled systems. Their drug release rates are controlled by factors like membrane permeability, drug solubility, and diffusivity.
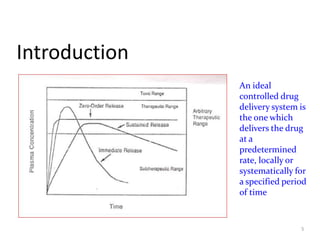
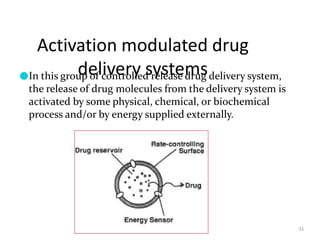
3. Activation modulated systems have drug release activated by physical, chemical, or biochemical processes. Examples described are osmotic pressure activated and vapor pressure activated systems which use osmotic or vapor pressure gradients to control drug release rates.



























![Sonophoresis- activated drug
delivery systems
52
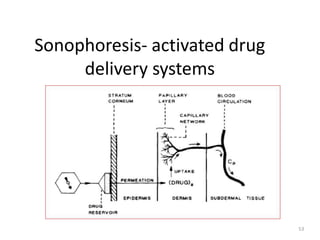
●Also called as Phonophoresis.
●This type of system utilizes ultrasonic energy to activate or
trigger the delivery of drug from polymeric drug delivery
device.
●System can be fabricated from nondegradable polymer
(ethylene vinyl acetate) or bioerodiable polymer
(poly[bis(p-carboxyphenoxy) alkane anhydride]
●The potential application of sonophoresis to regulate the
delivery of drugs was recently reviewed.](https://image.slidesharecdn.com/ratecontrolledddssips-240120090328-81280342/85/Rate-controlled-DDS-SIPS-pptx-52-320.jpg)