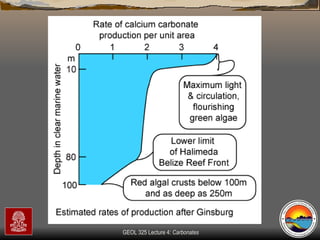
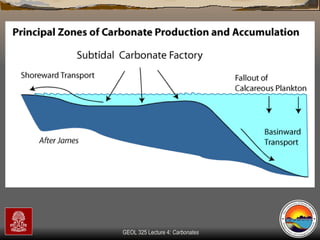
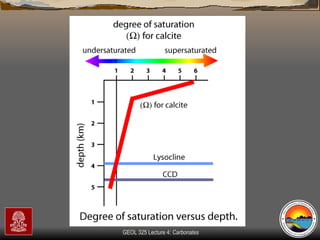
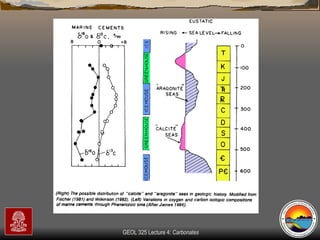
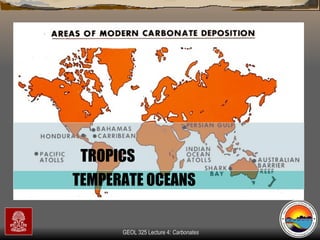
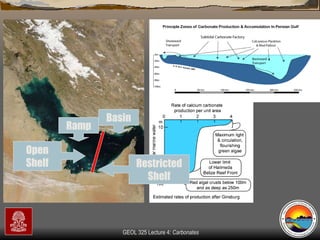
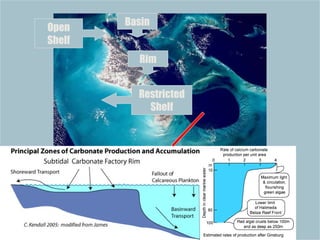
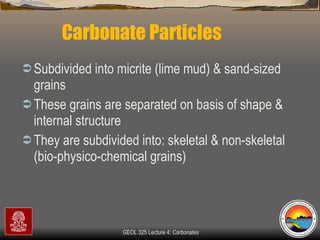
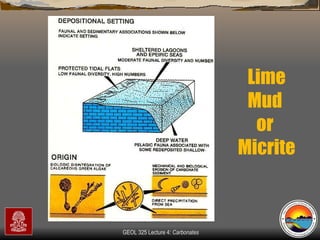
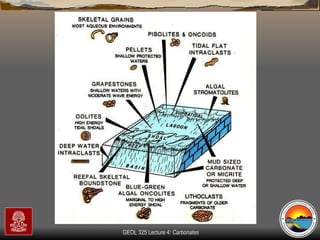
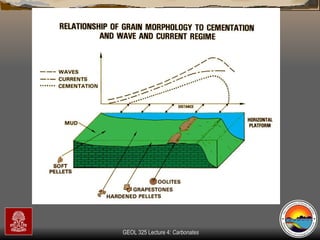
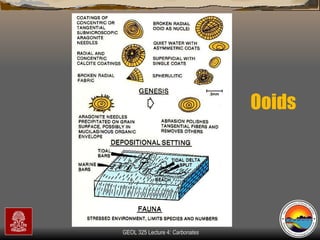
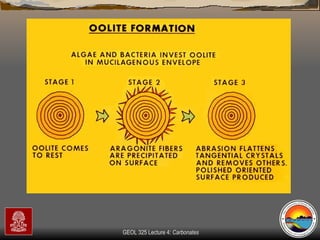
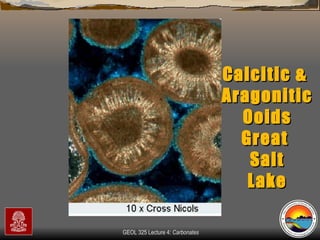
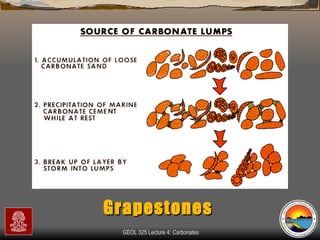
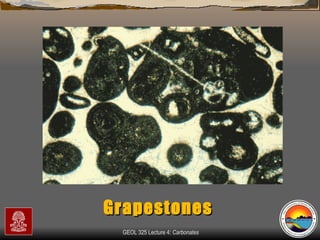
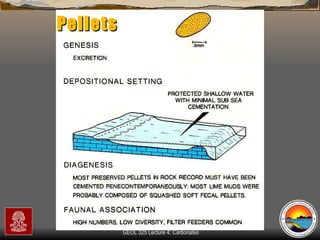
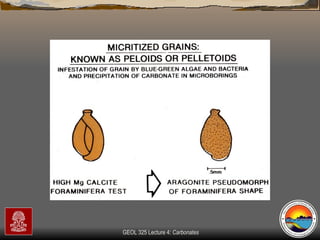
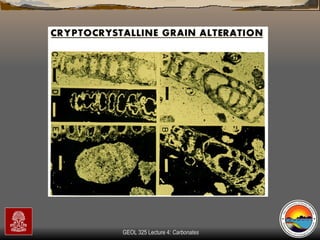
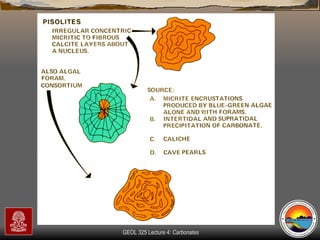
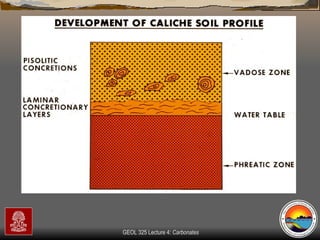
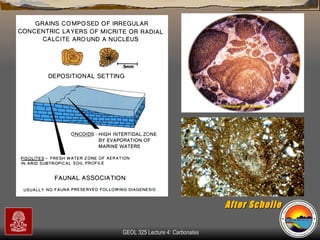
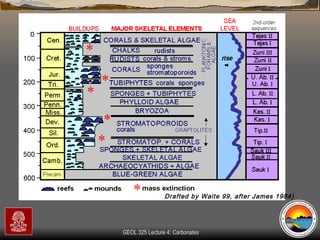
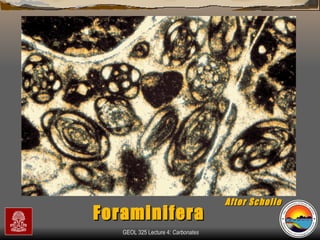
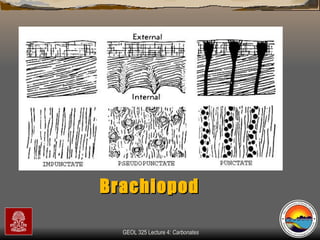
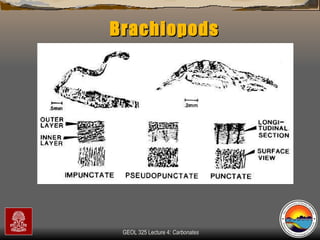
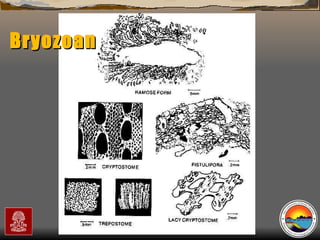
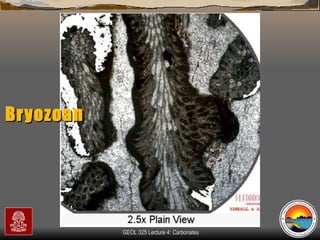
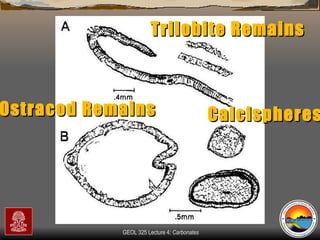
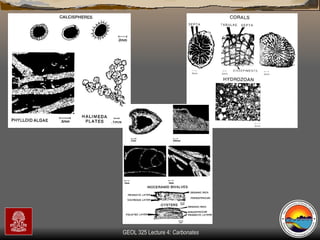
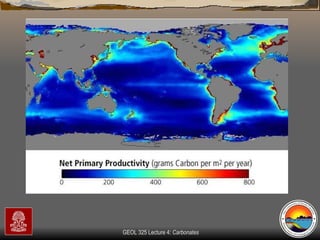
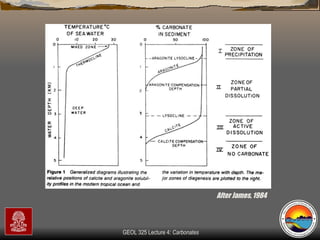
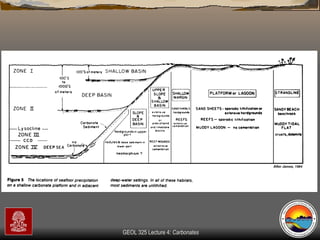
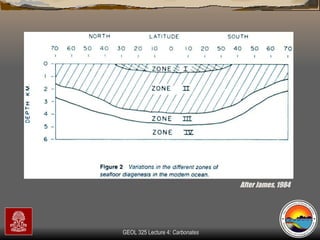
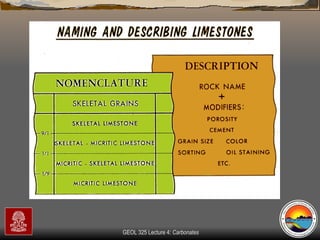
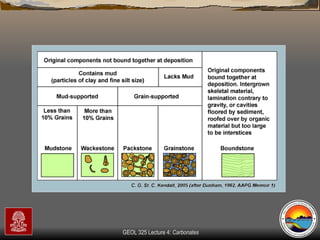
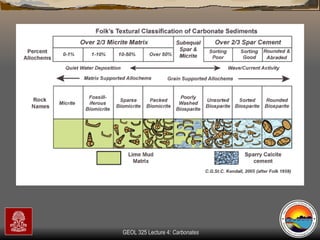
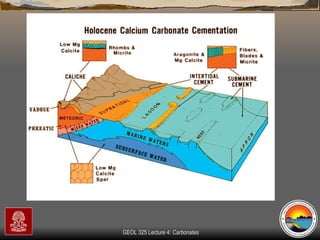
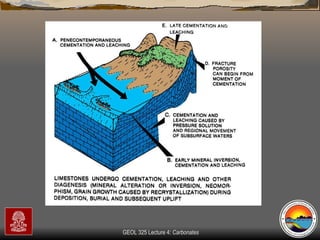
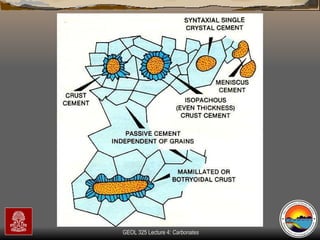
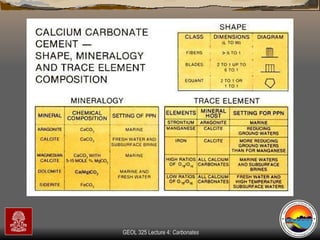
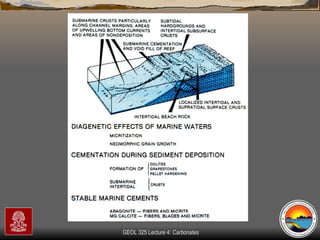
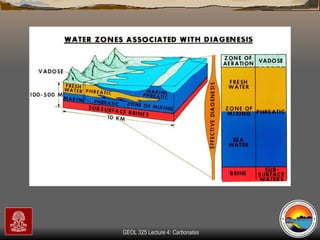
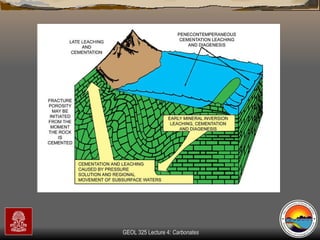
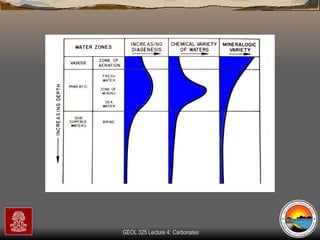
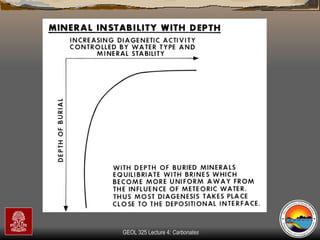
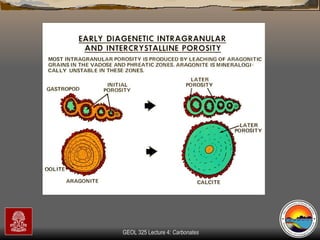
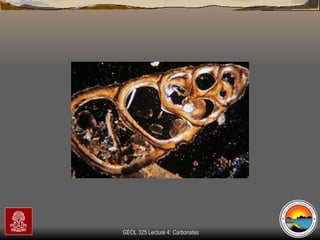
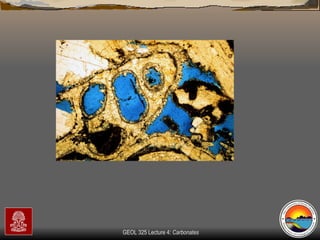
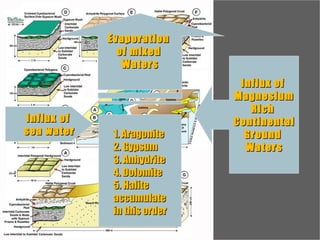
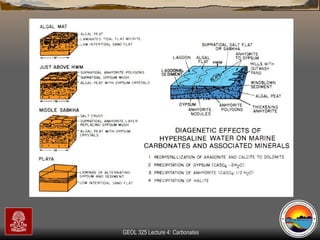
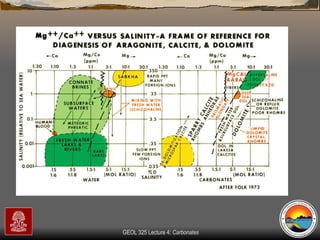
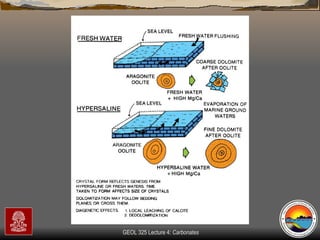
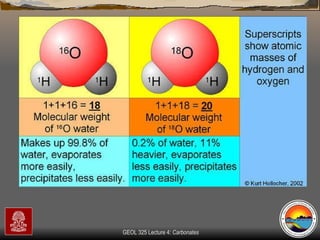
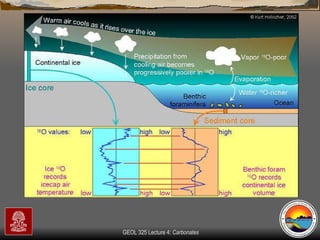
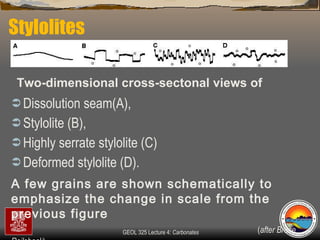
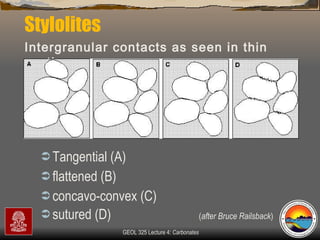
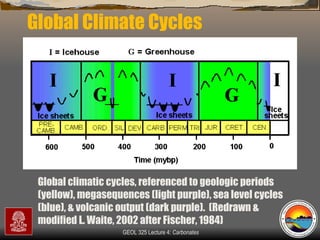
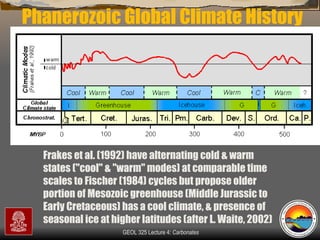
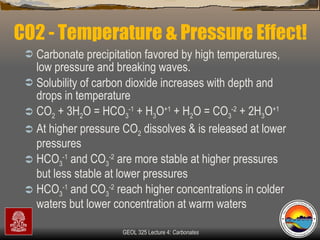
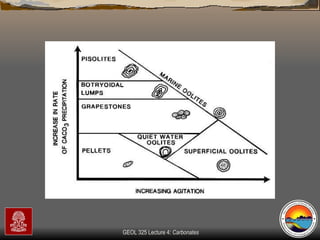
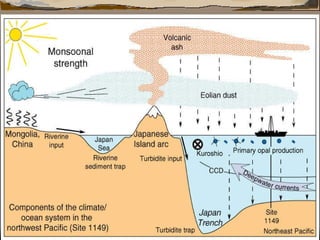
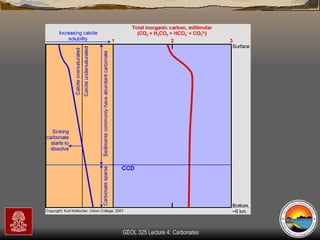
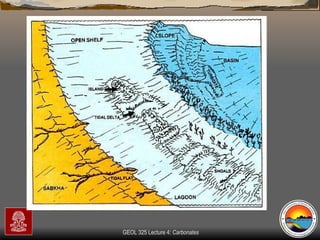
The document provides an overview of a course on carbonates and sedimentary basins. It discusses how carbonate sediments form in different depositional environments and the factors controlling carbonate accumulation and distribution. It also summarizes the key components and textures of carbonate rocks and the processes of diagenesis.