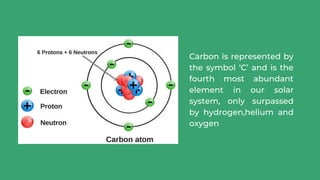
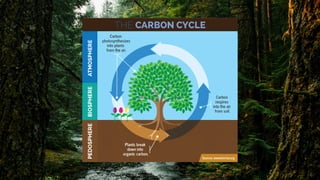
Carbon is a nonmetal element that is essential to life on Earth and is continuously cycled through the atmosphere, biosphere, oceans, and Earth's crust. It forms the basis of all living organisms and is a key component in regulating the planet's climate. The carbon cycle describes how carbon continuously moves between the atmosphere, living things, oceans, and Earth's crust through natural processes like respiration, photosynthesis, and volcanic eruptions. However, human activities like burning fossil fuels have increased the amount of carbon dioxide in the atmosphere, disrupting the natural carbon cycle and causing climate change.