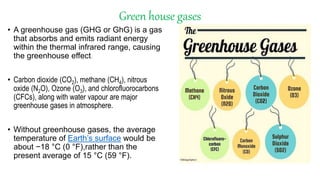
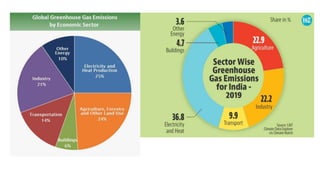
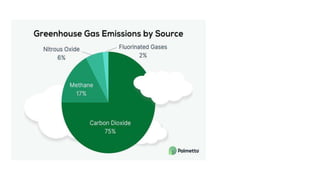
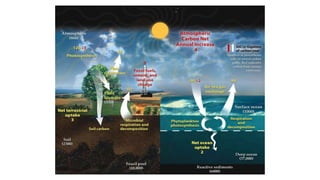
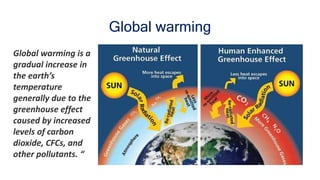
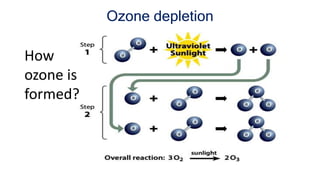
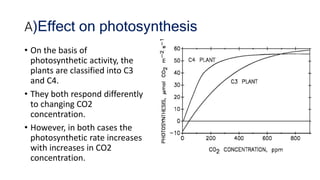
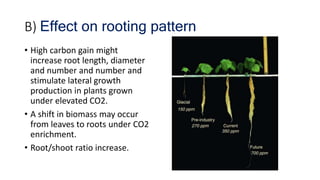
Greenhouse gases like carbon dioxide and methane absorb and emit radiation within the Earth's atmosphere, causing the greenhouse effect which maintains a habitable average temperature of 15°C. Natural sources include respiration and volcanic eruptions, but human activities like burning fossil fuels and deforestation have increased greenhouse gas levels. Plants and soils act as carbon sinks by absorbing carbon dioxide through photosynthesis. Rising CO2 levels affect plant physiology and biochemistry differently for C3 and C4 plants, generally increasing photosynthesis but decreasing seed germination and food quality.