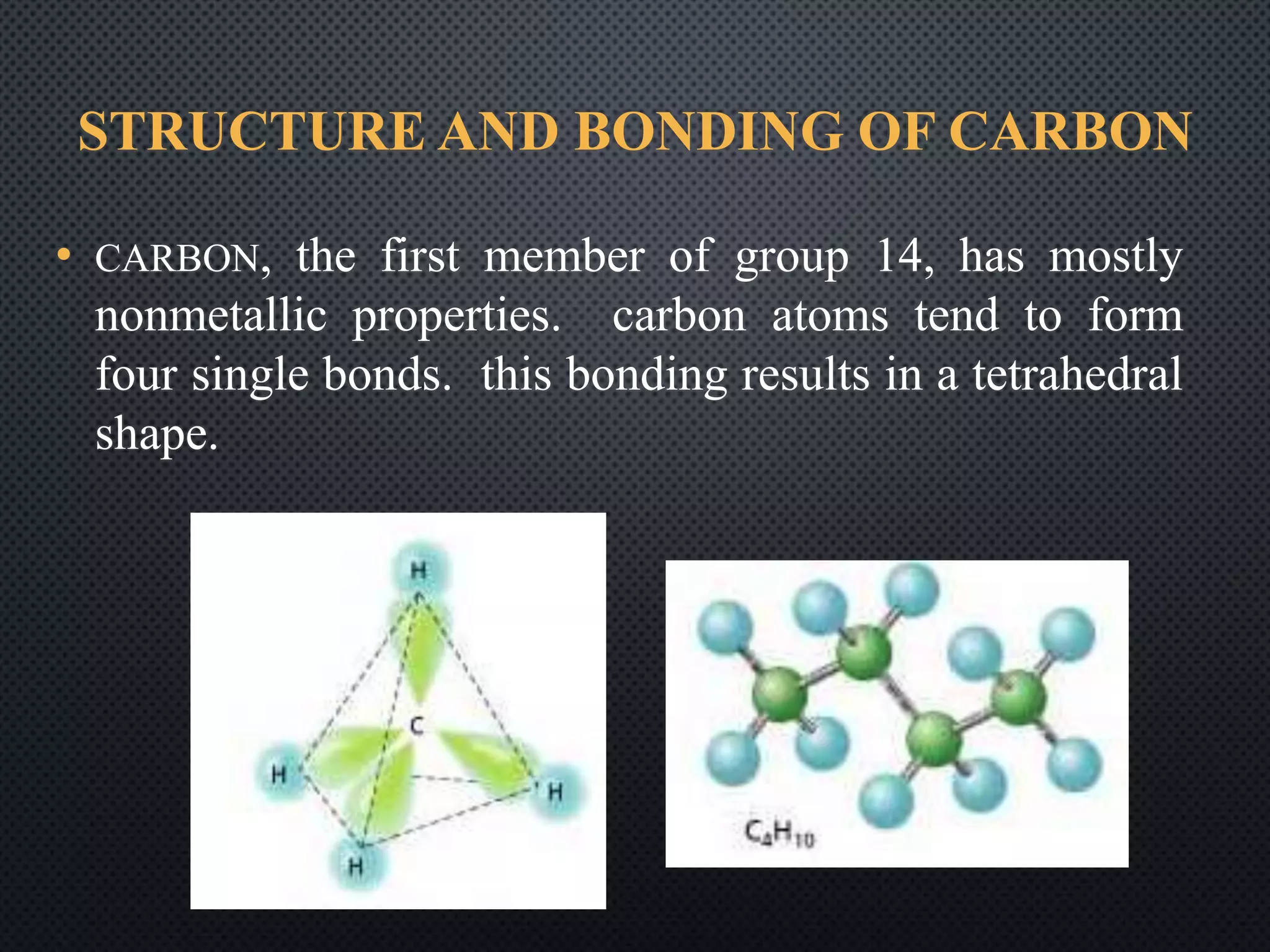
This document summarizes key topics relating to carbon and hydrocarbons, including:
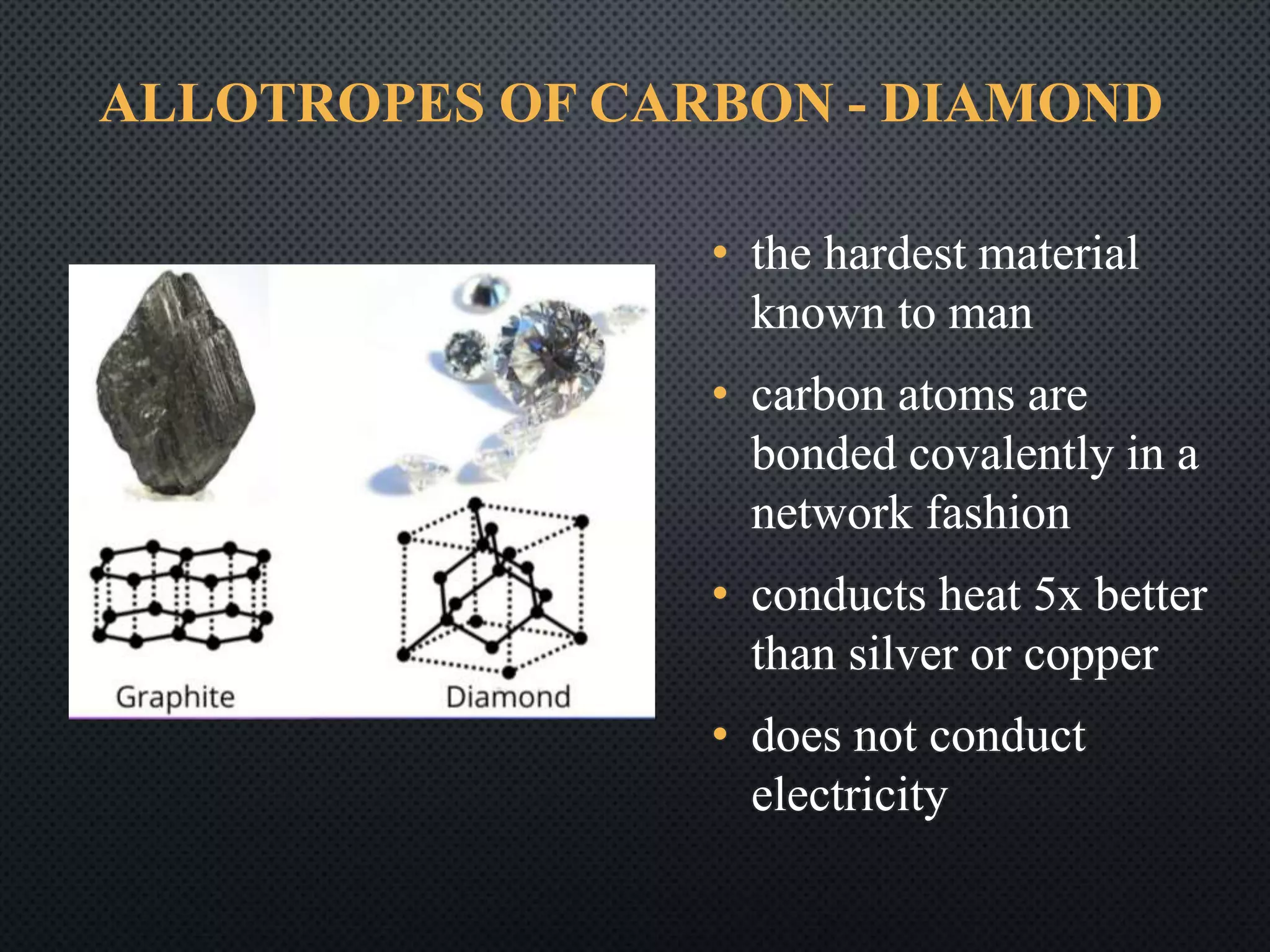
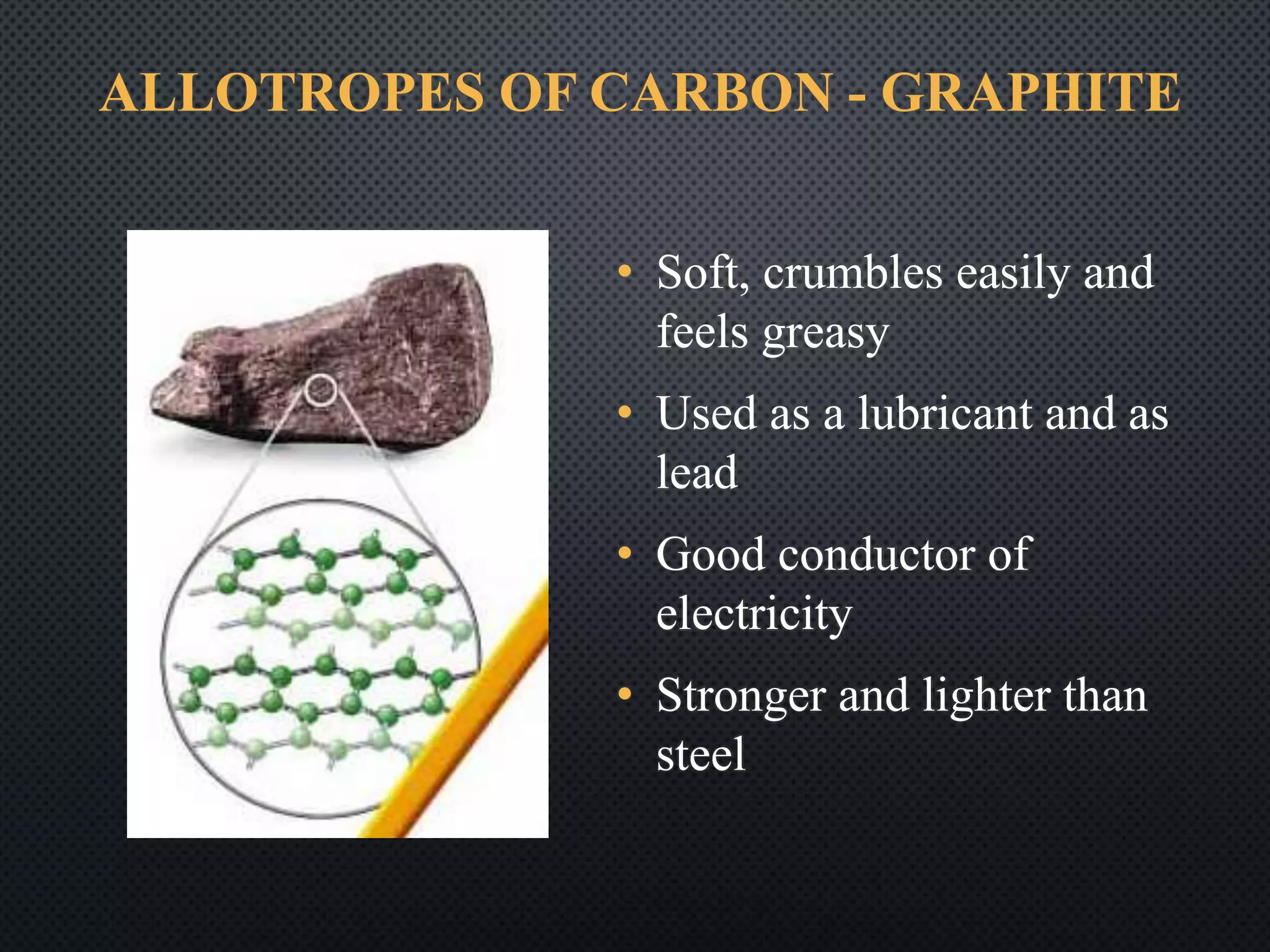
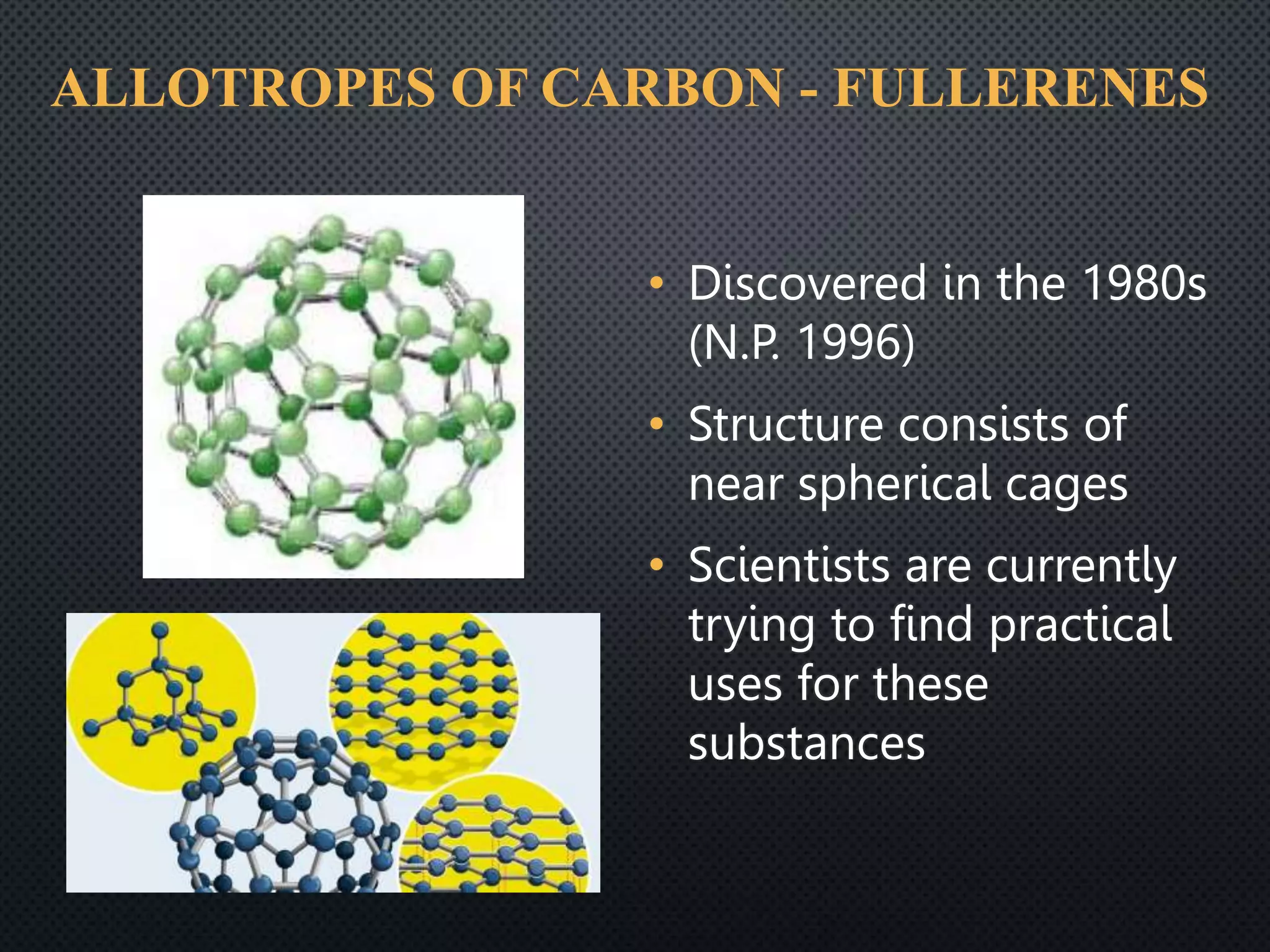
1) Carbon occurs in several allotropic forms including diamond, graphite, and fullerenes which have different properties; diamond is the hardest known material while graphite is soft and conducts electricity.
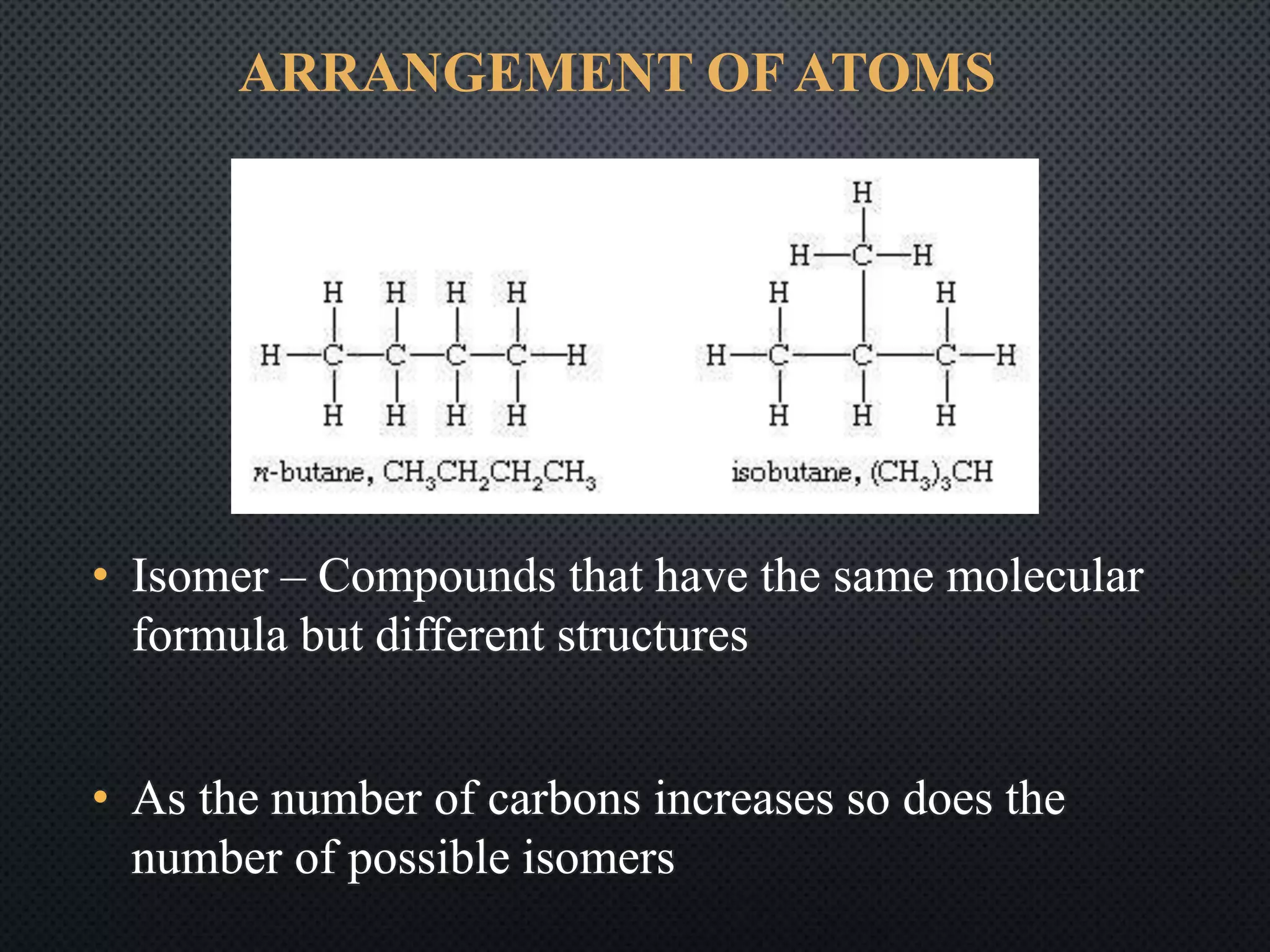
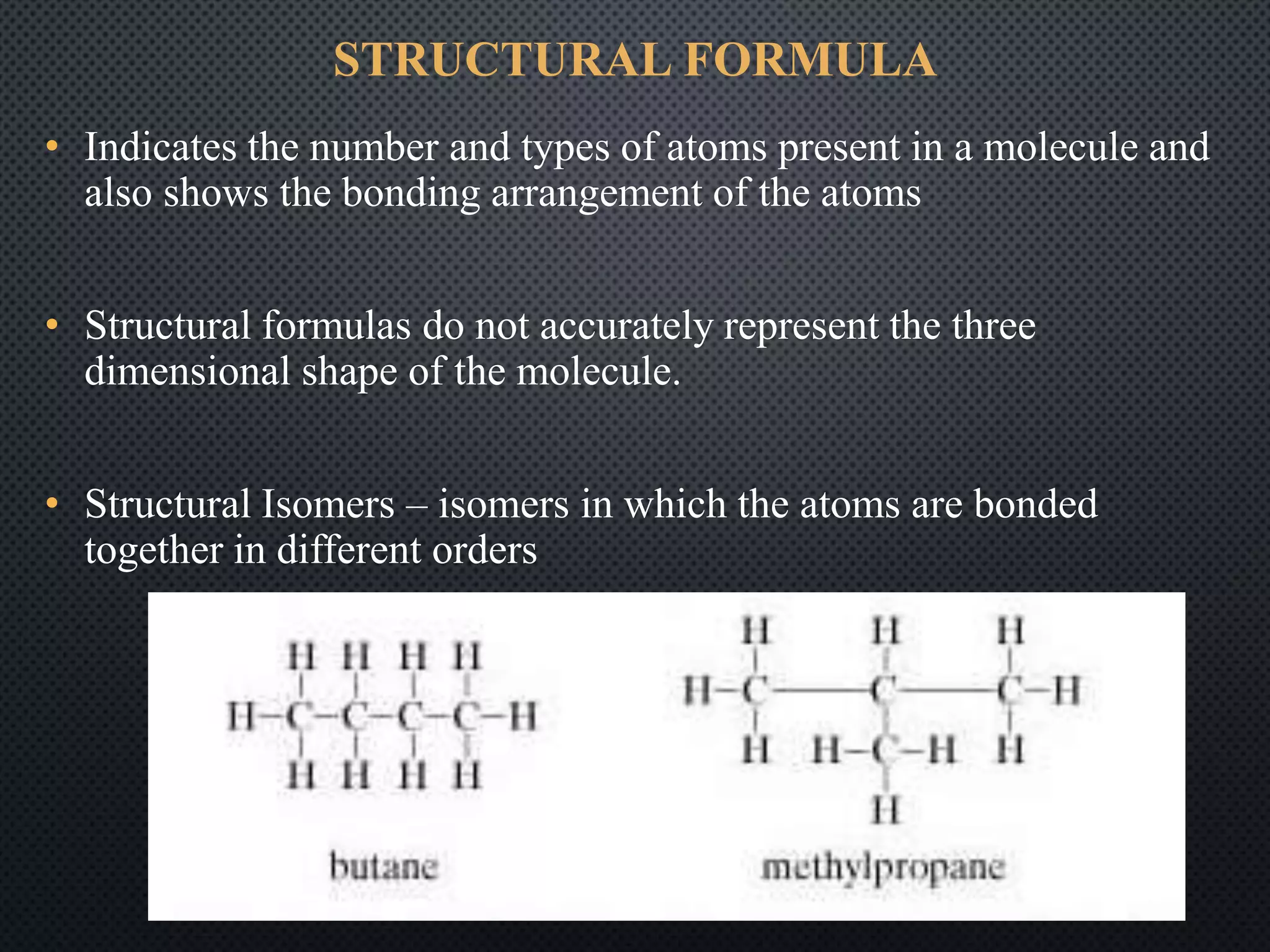
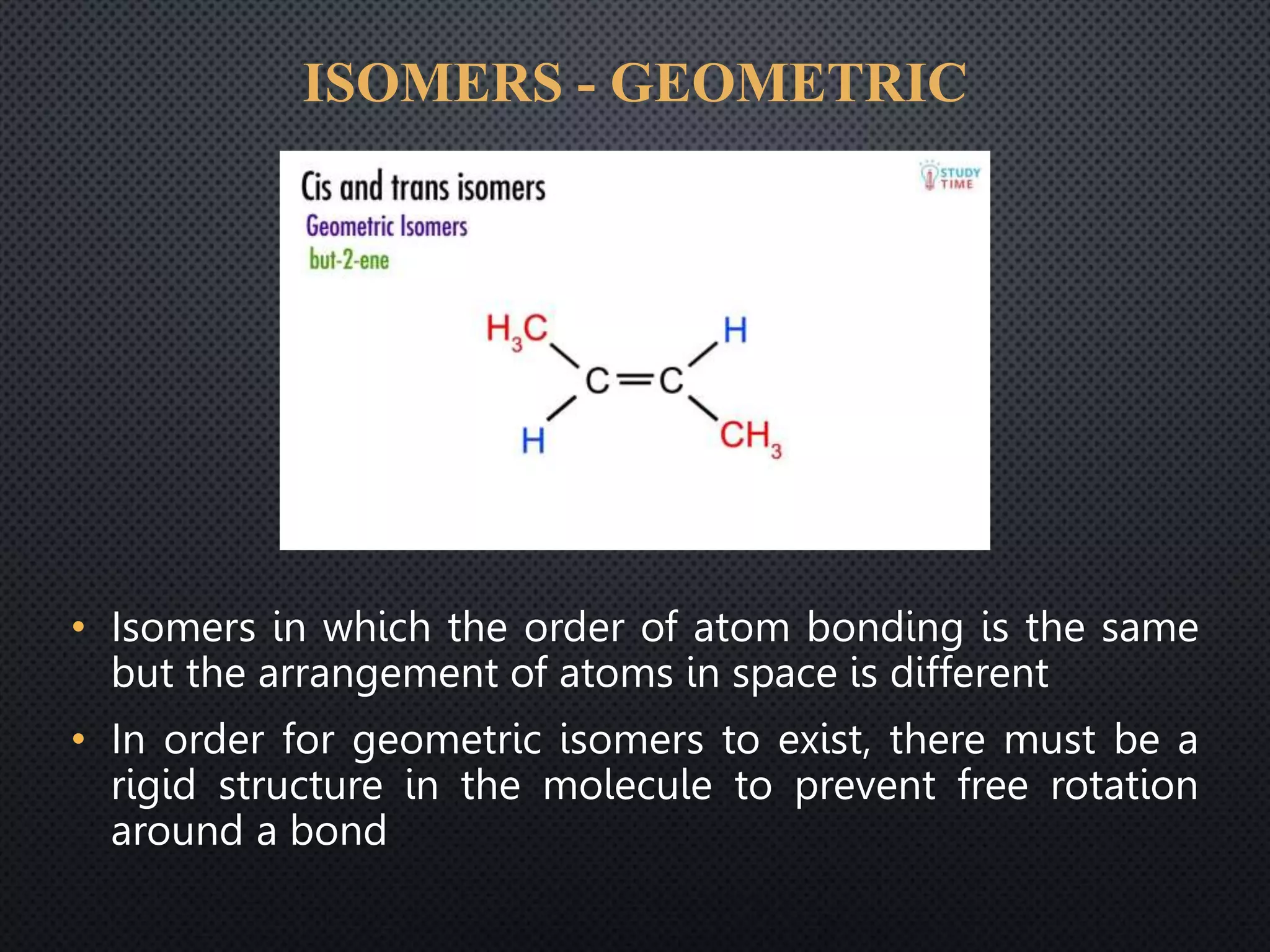
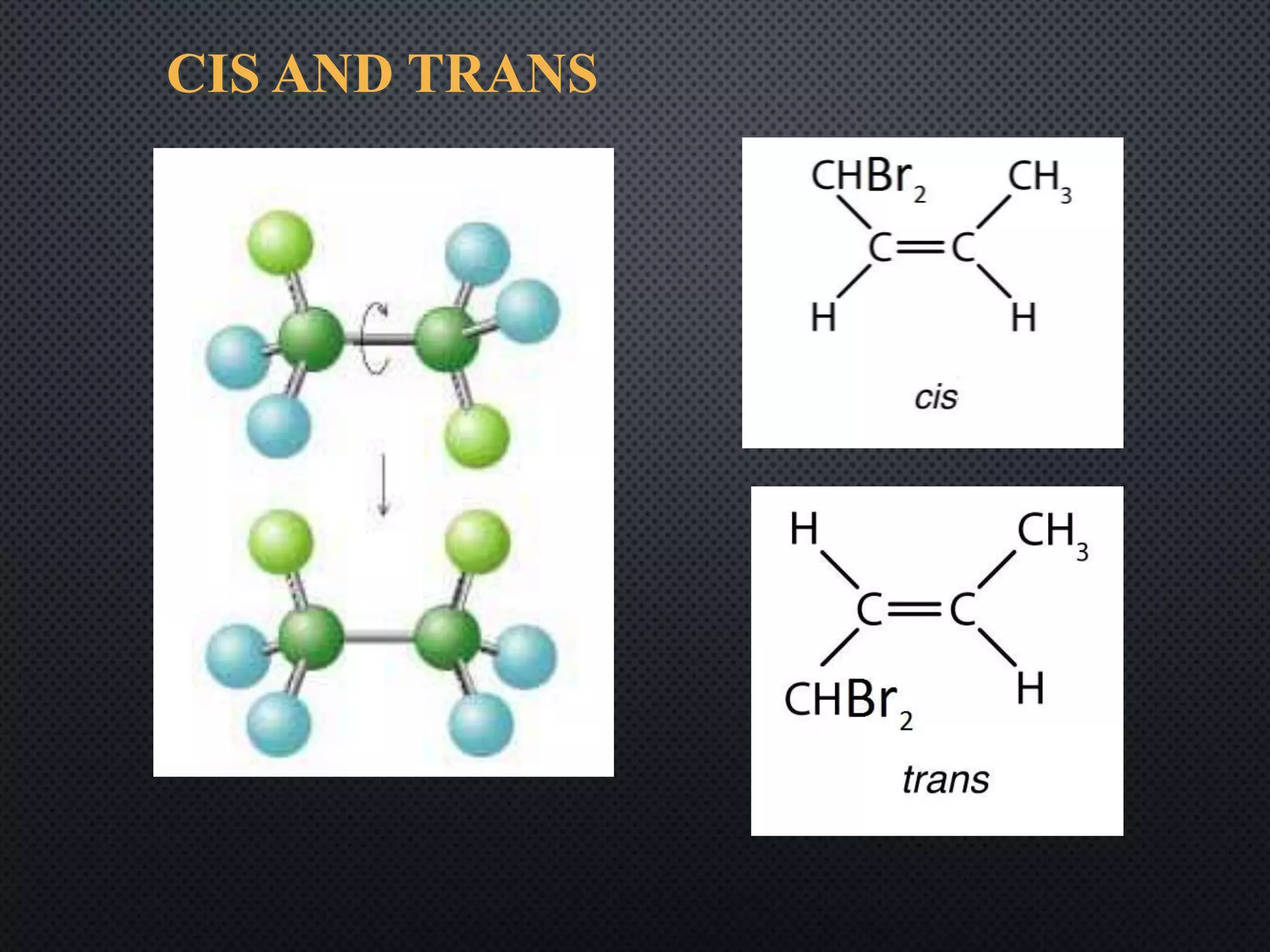
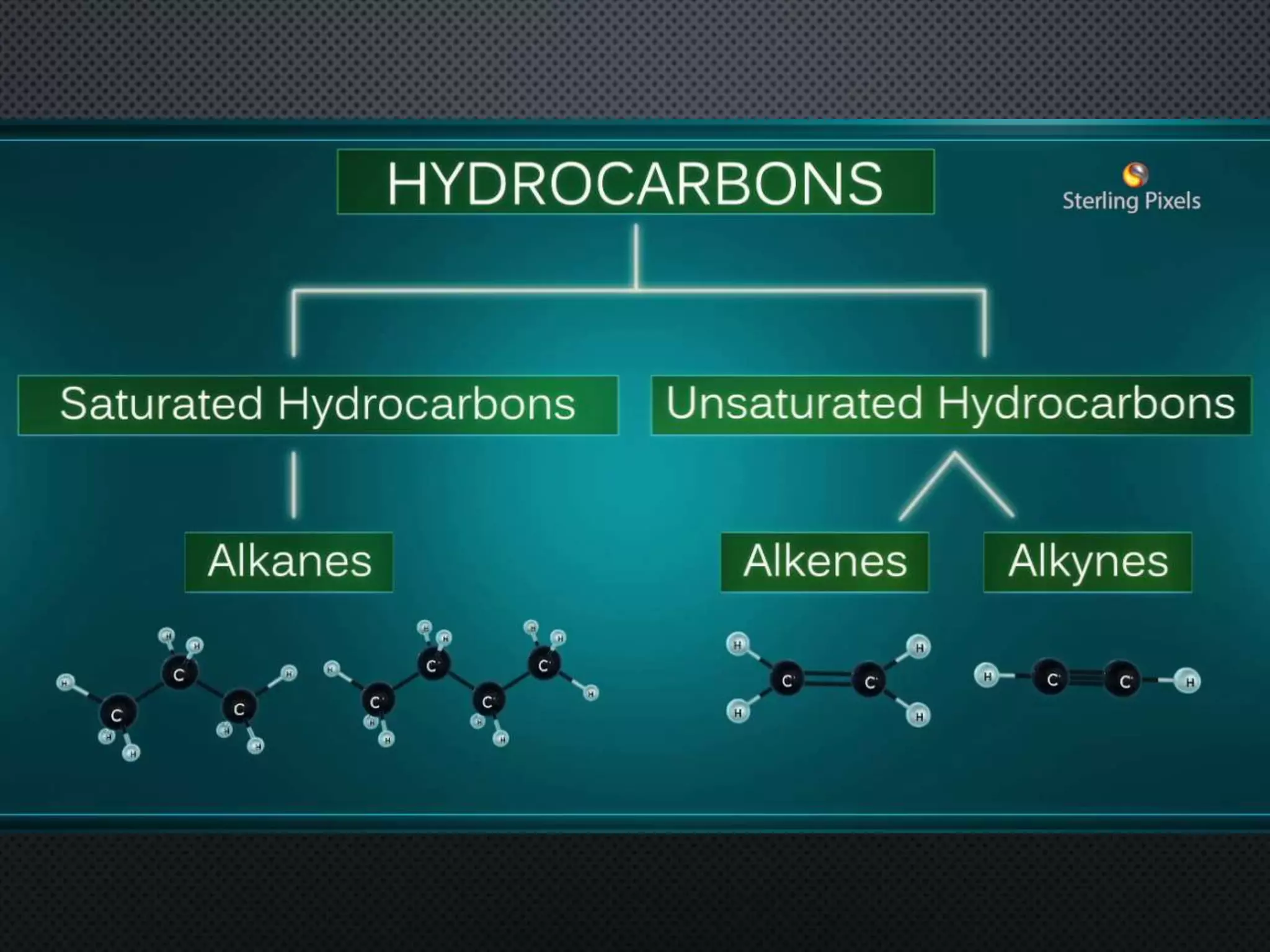
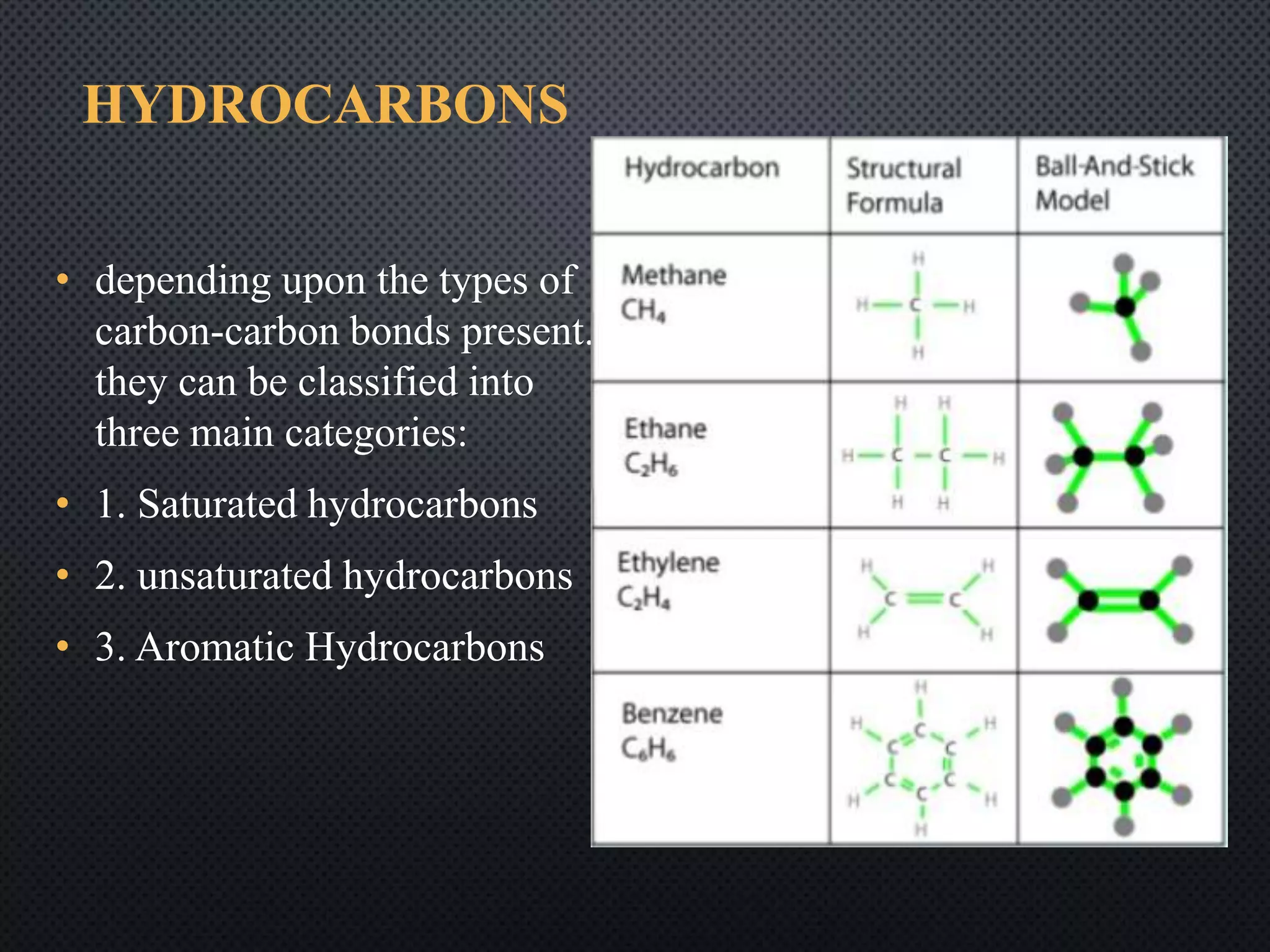
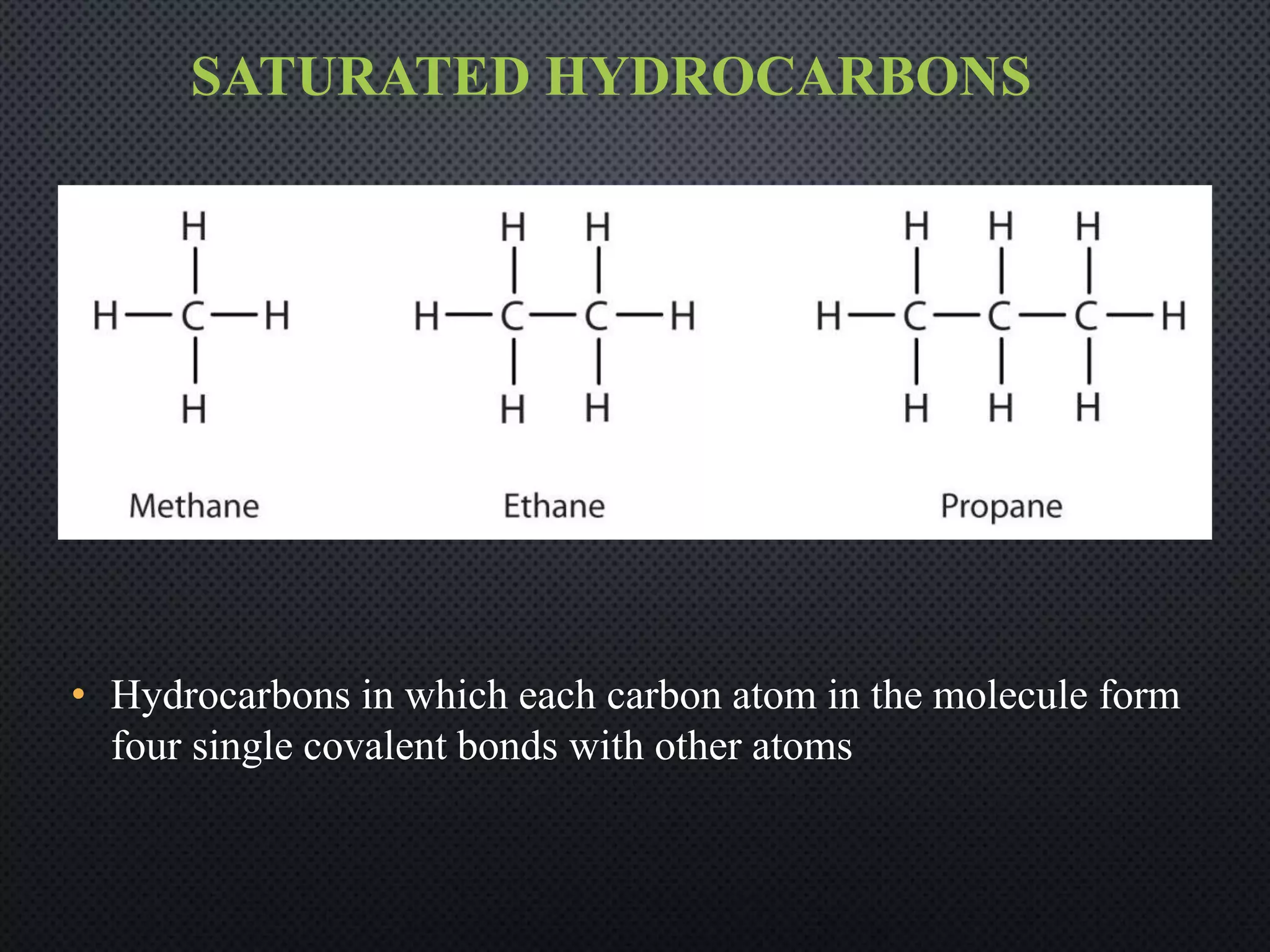
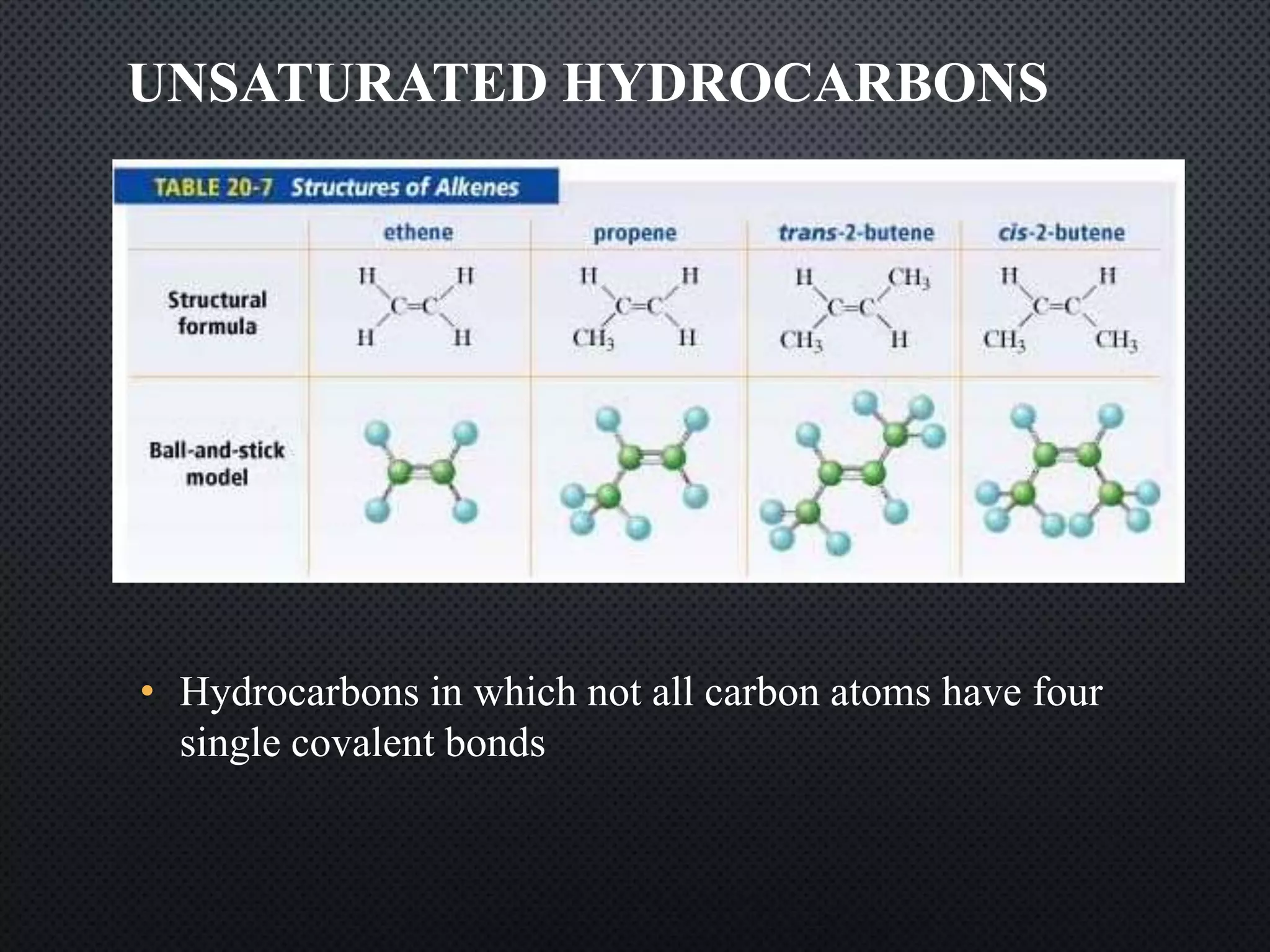
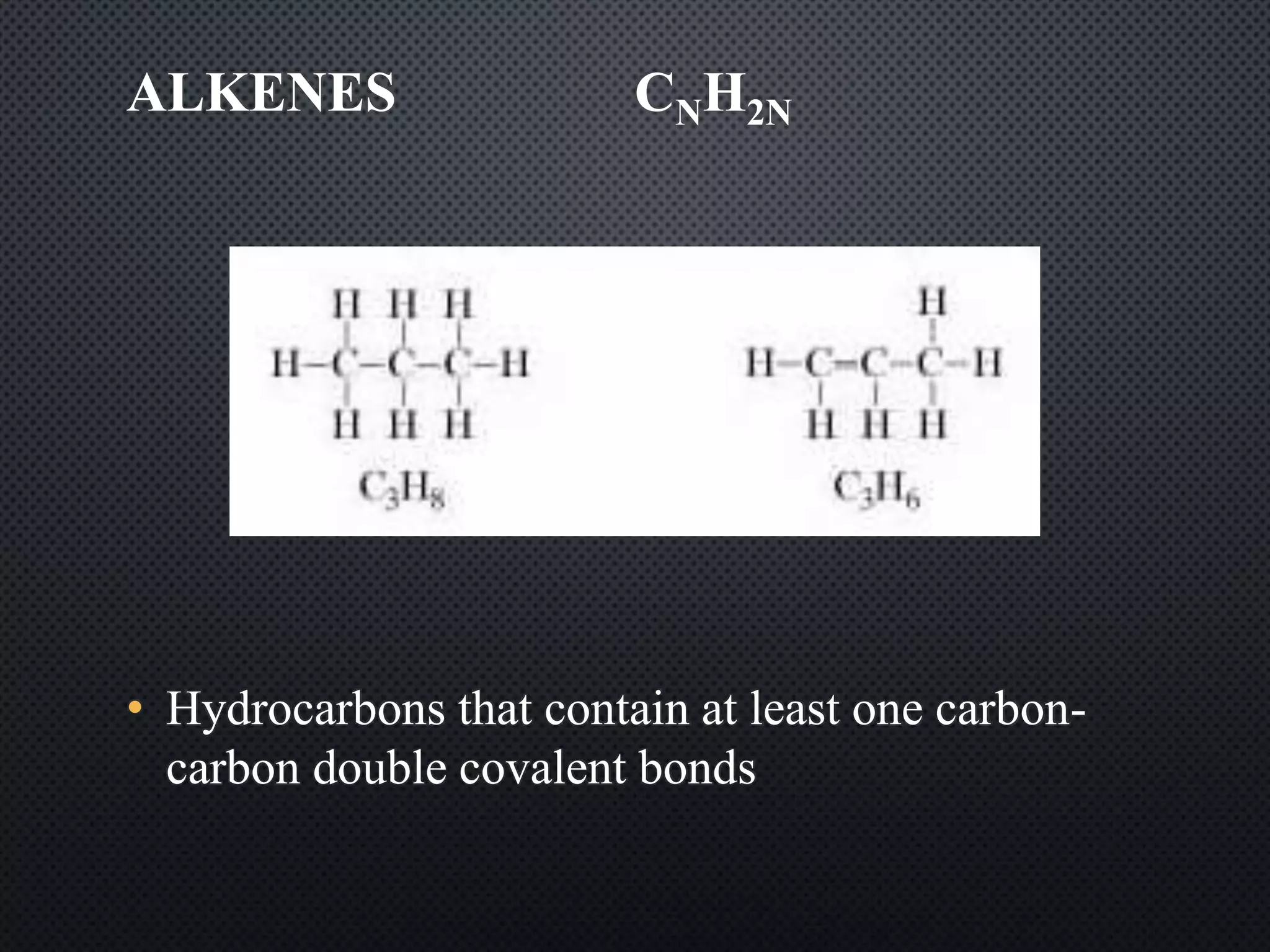
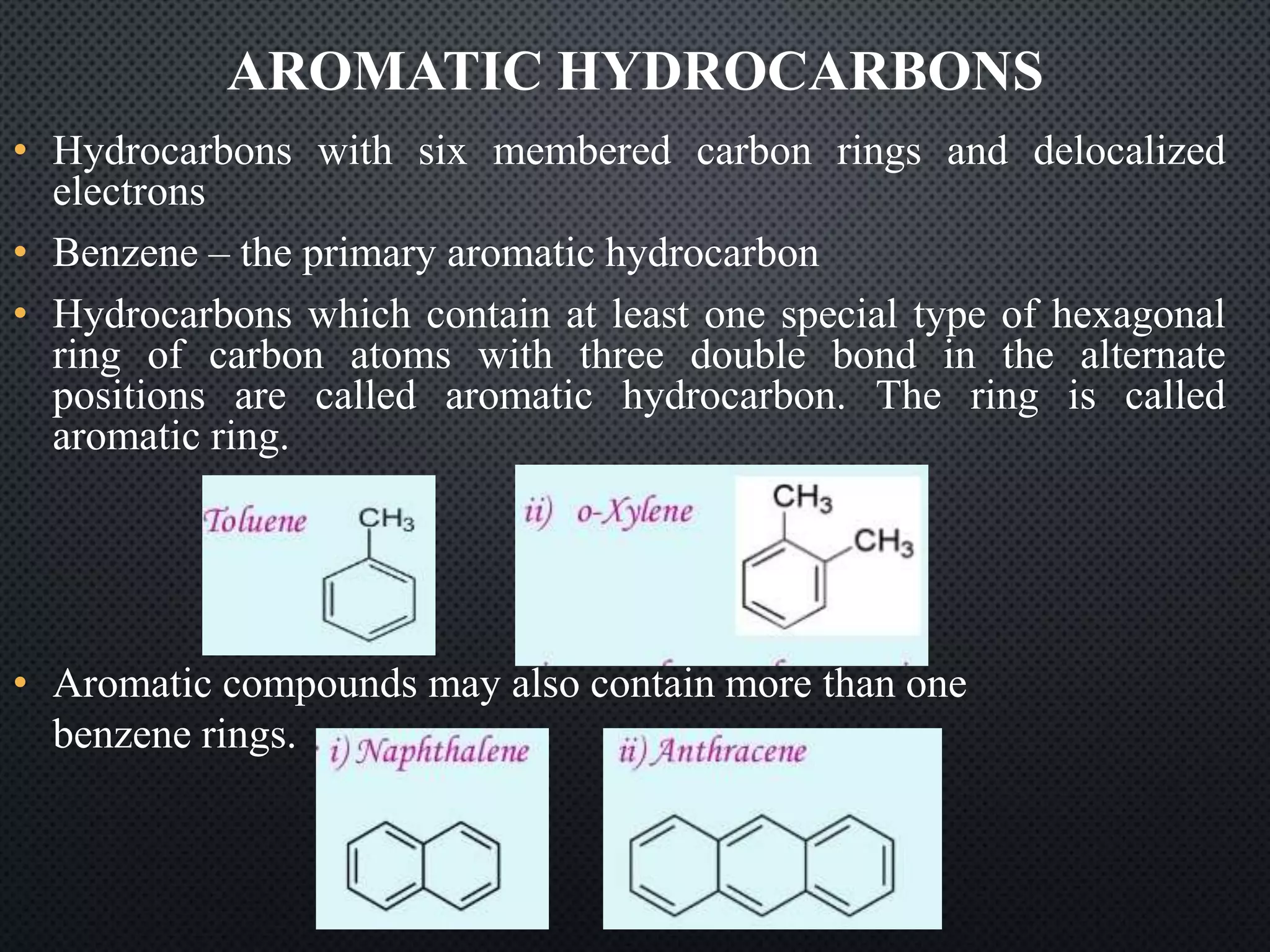
2) Hydrocarbons can be classified as saturated, containing only single bonds, unsaturated with double or triple bonds, or aromatic with delocalized electrons in ring structures.
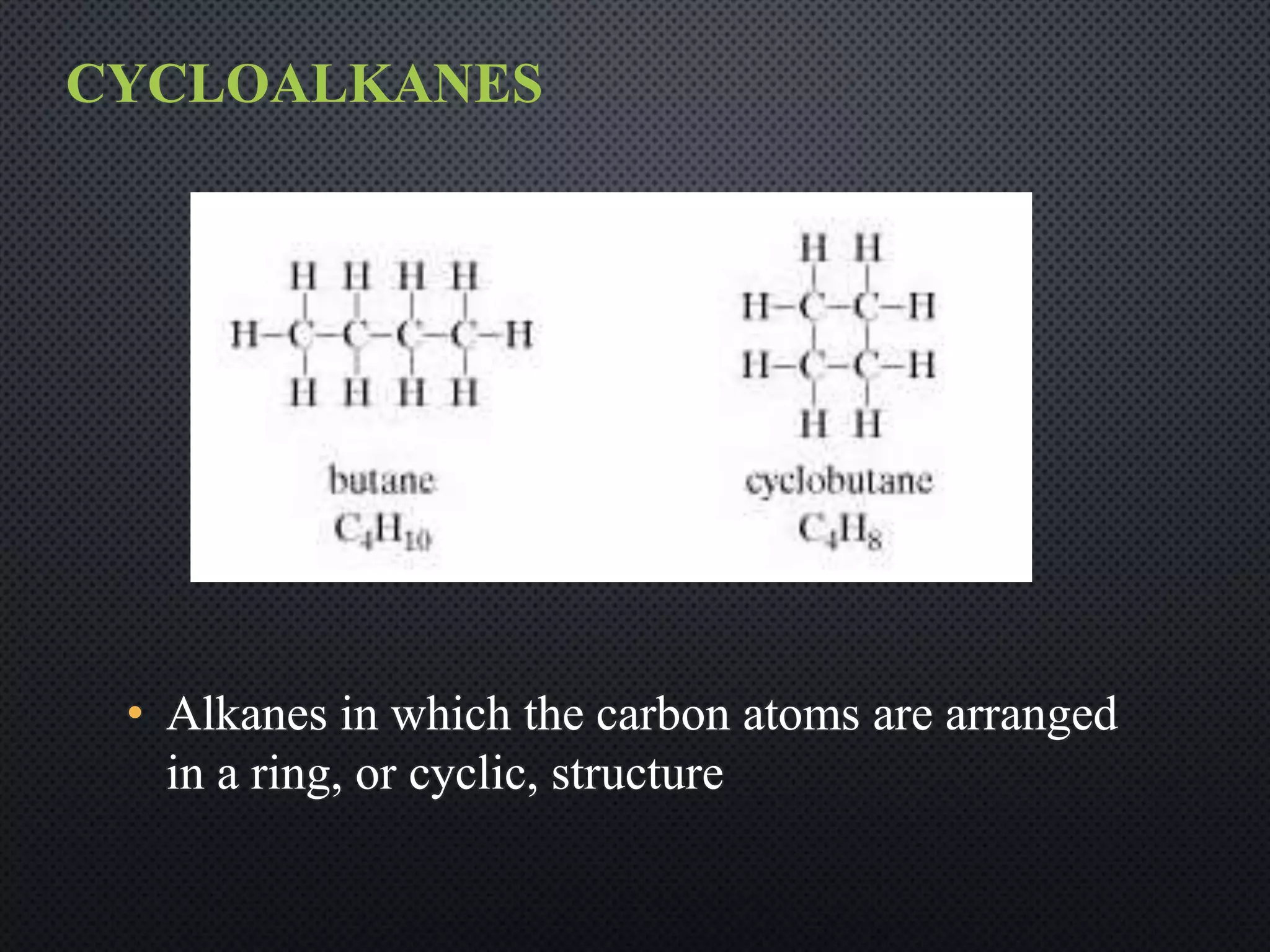
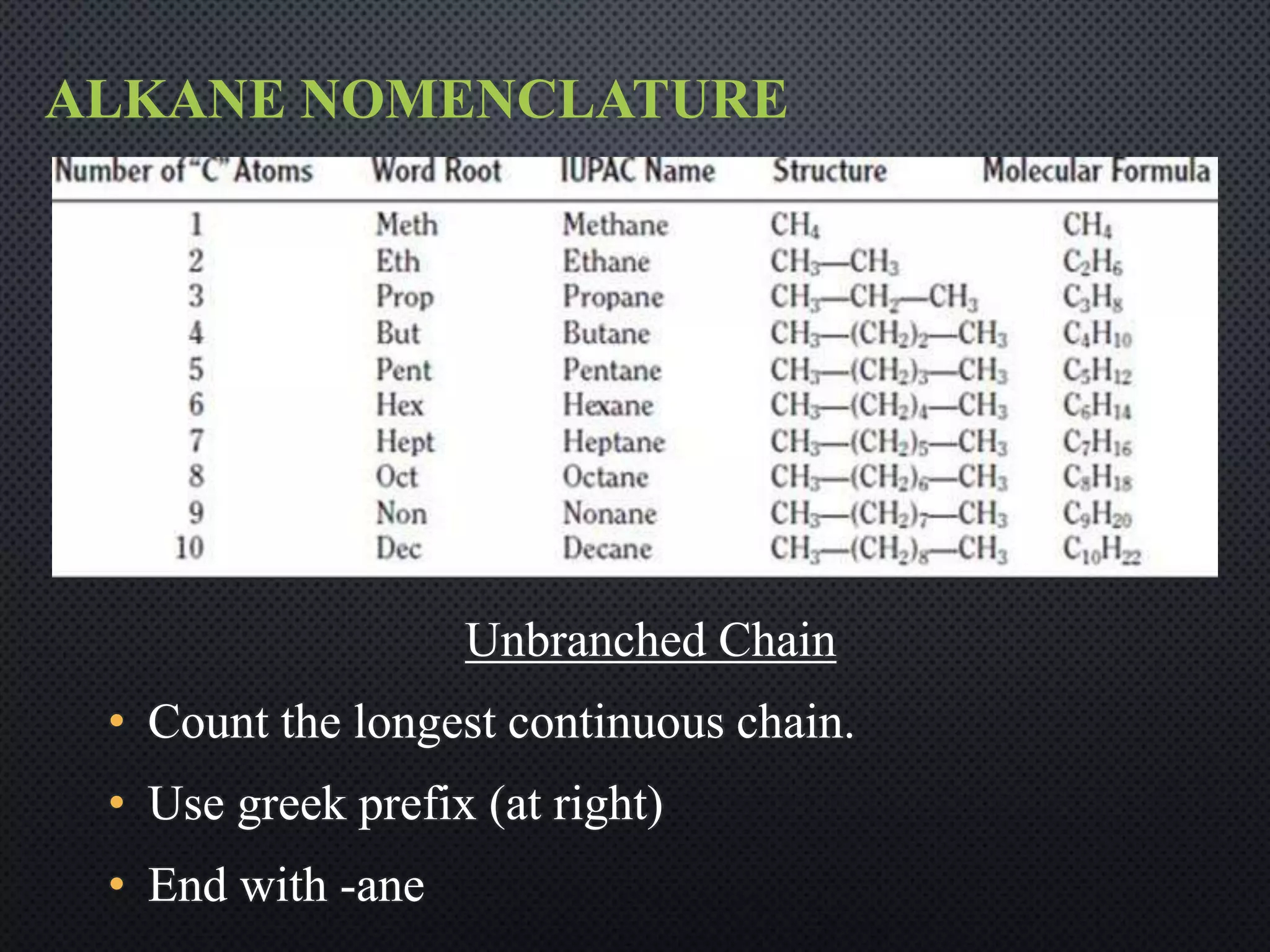
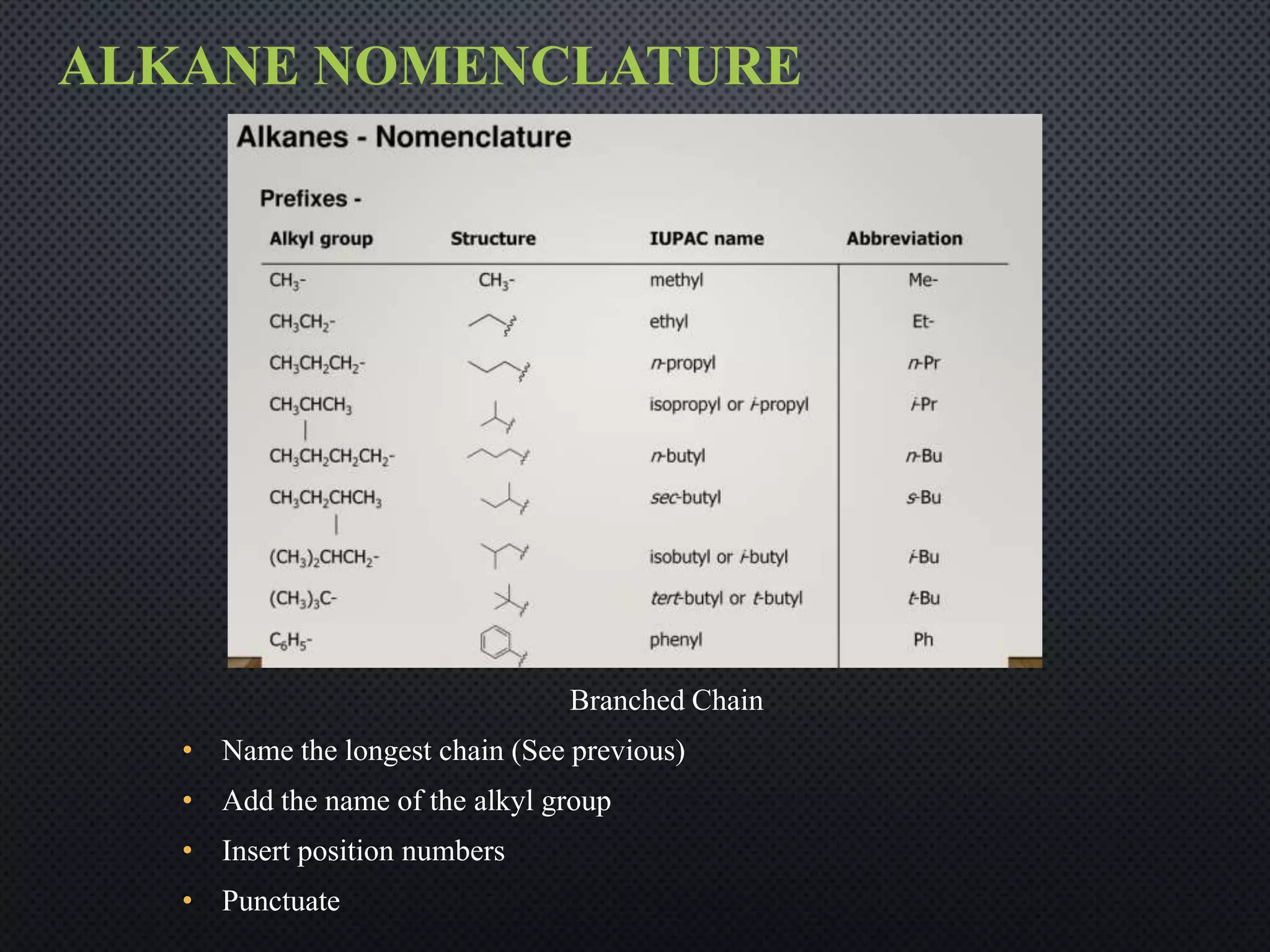
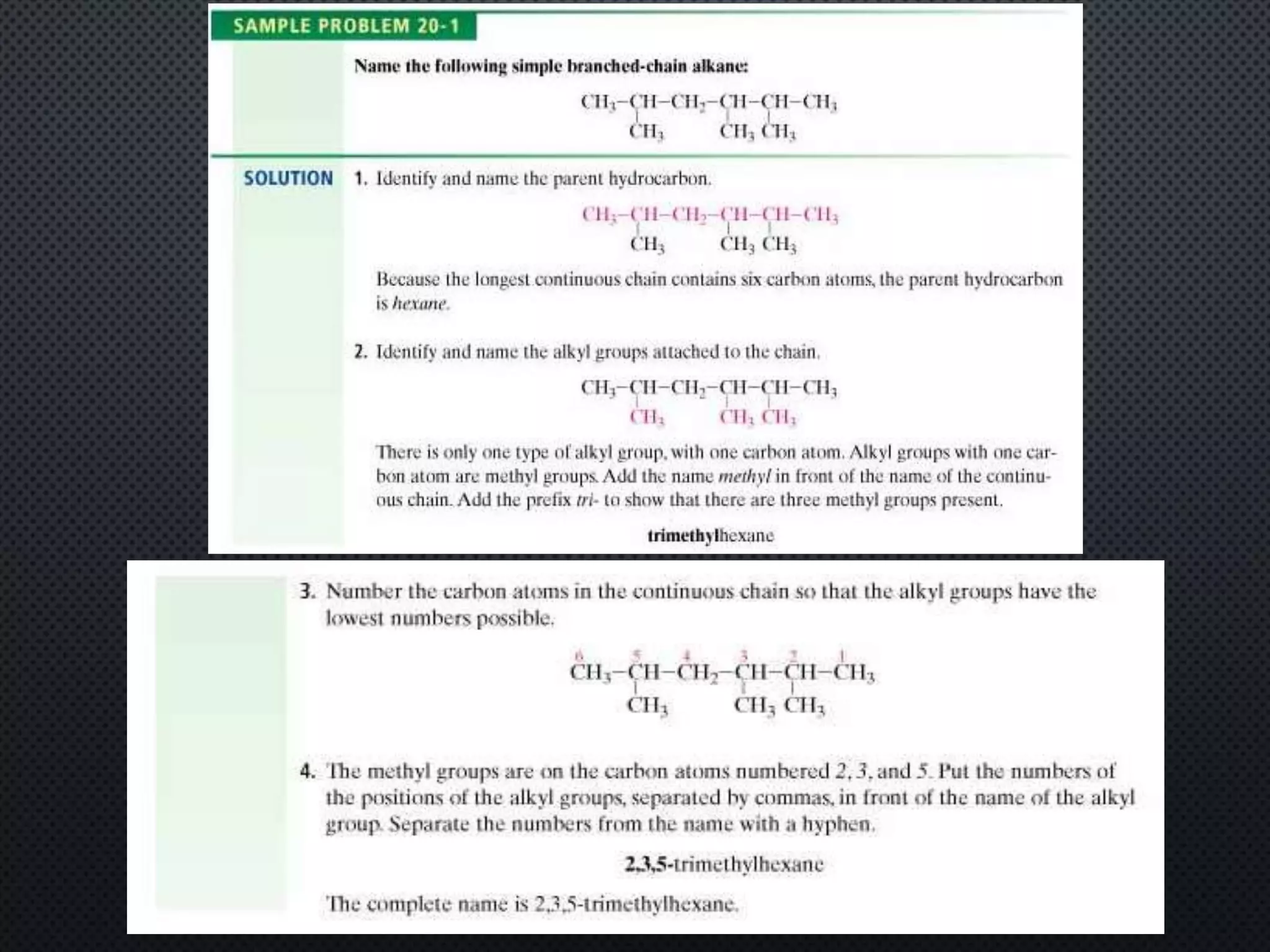
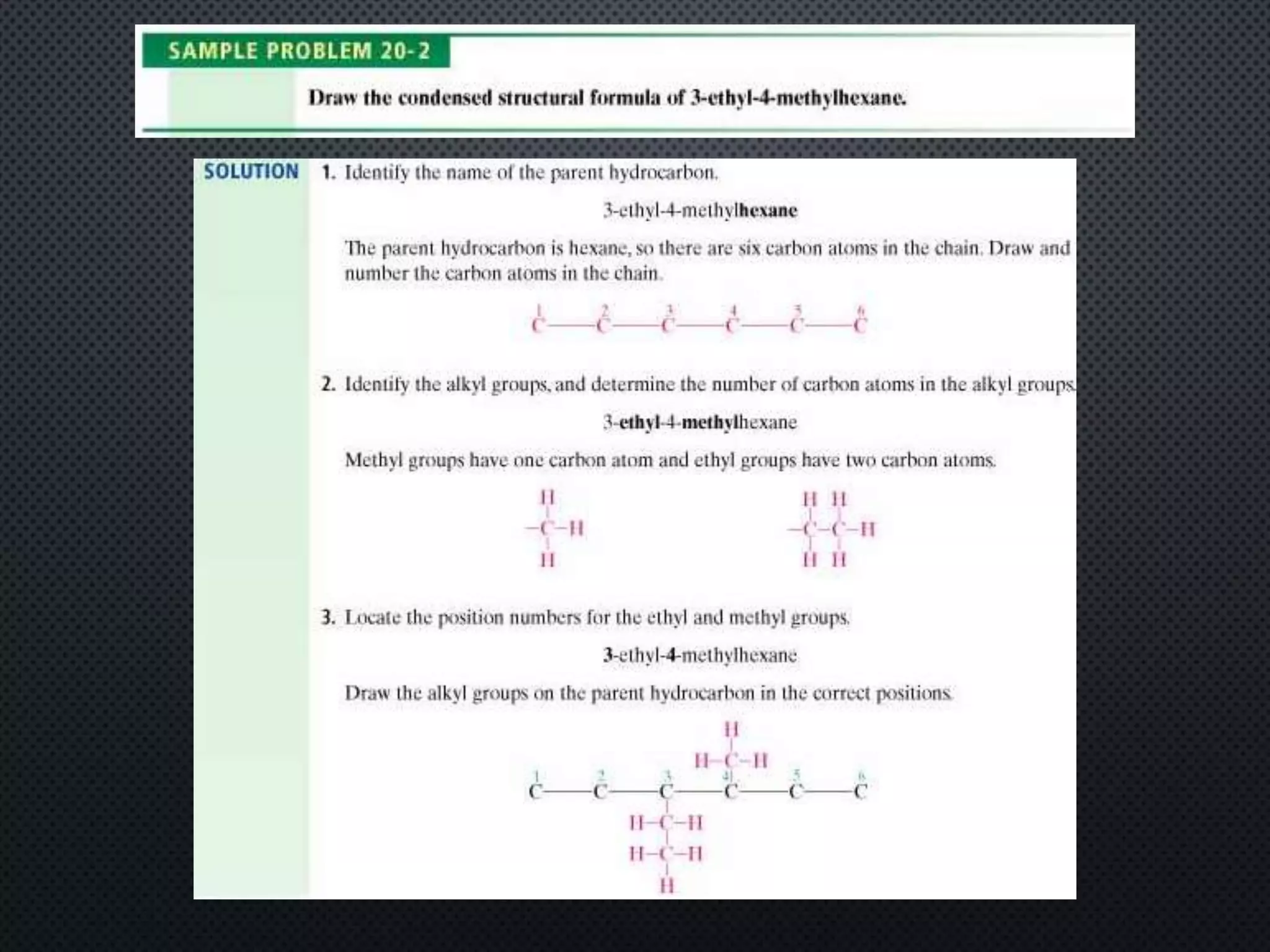
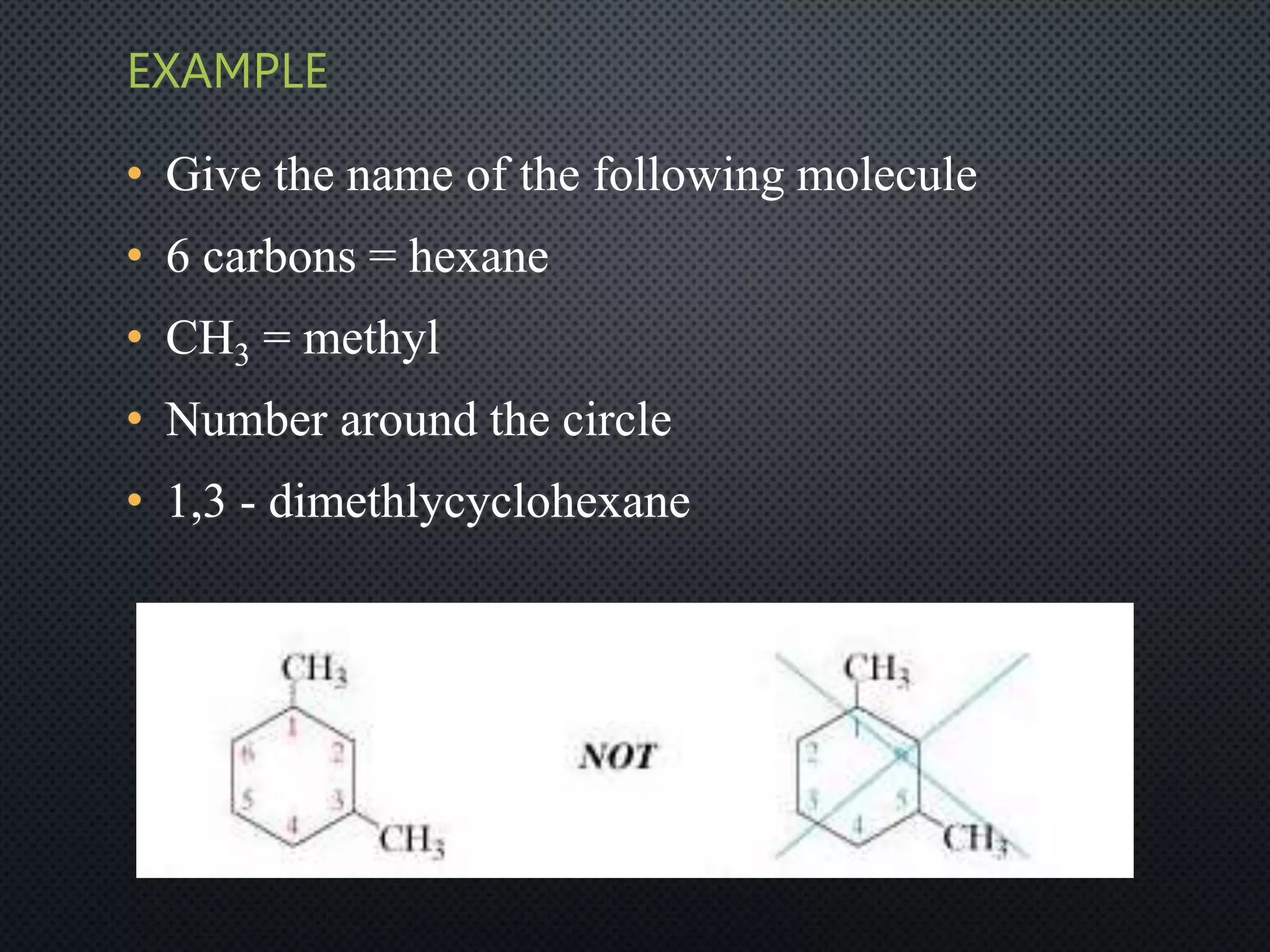
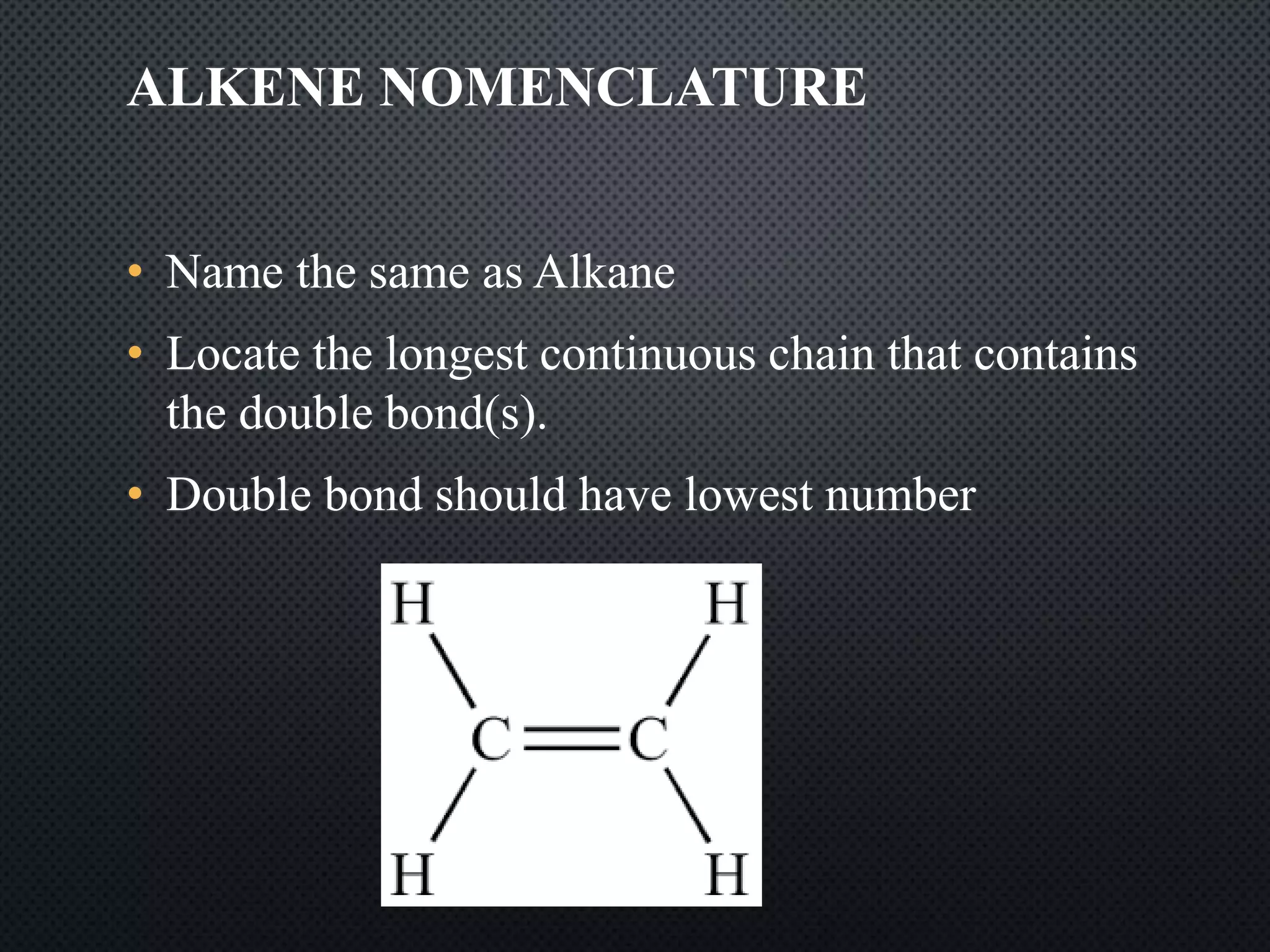
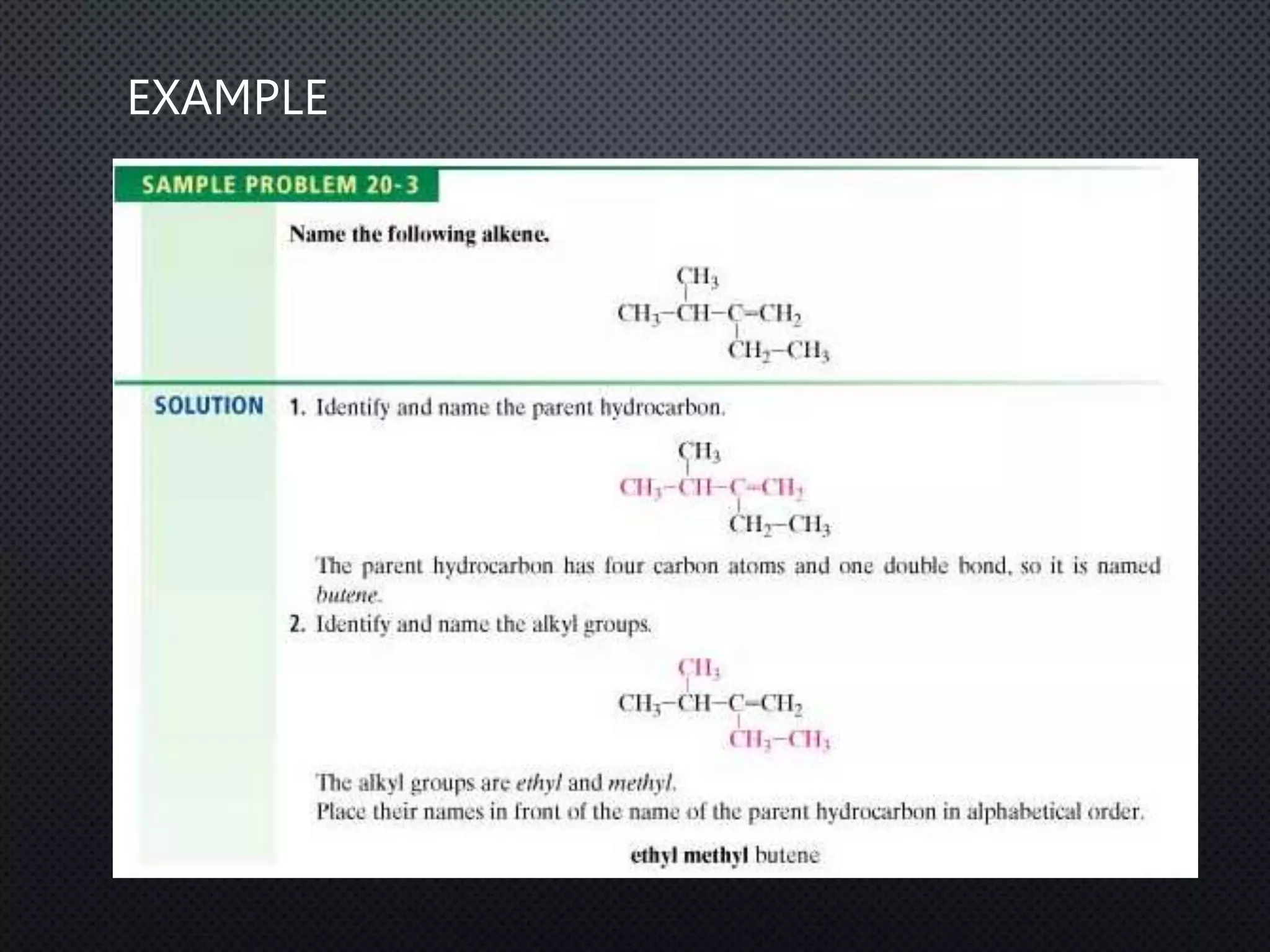
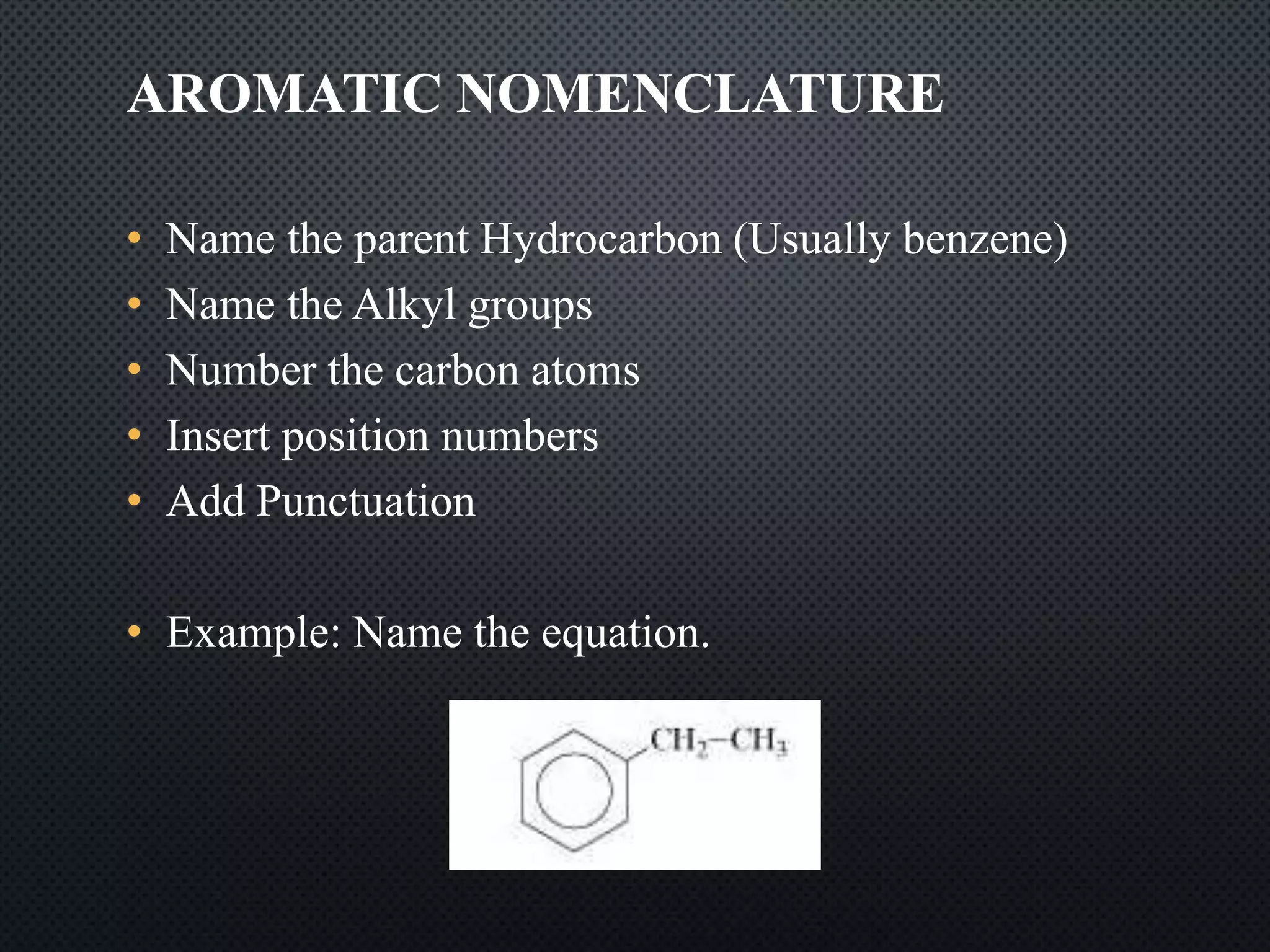
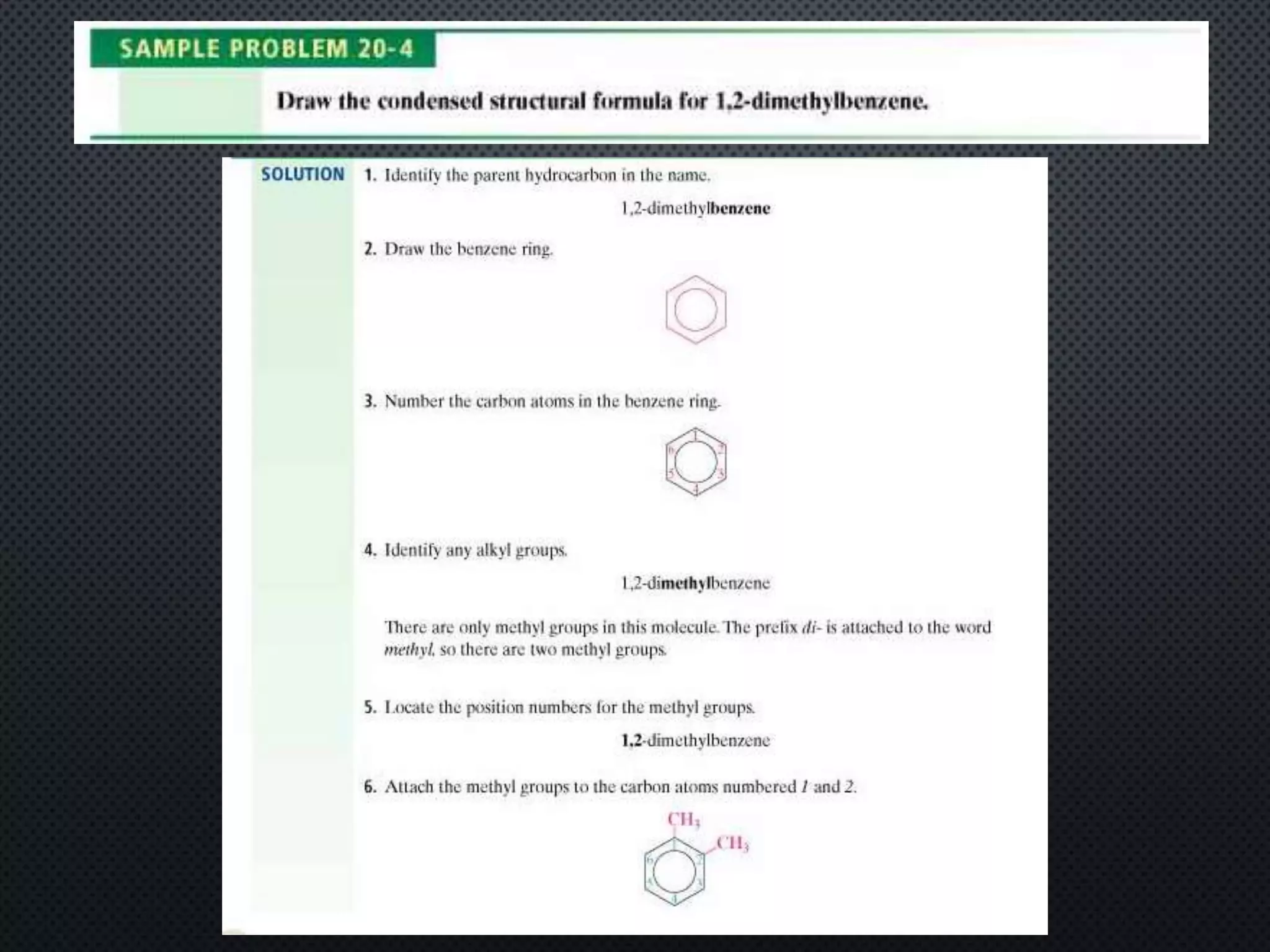
3) Nomenclature rules are provided for naming alkanes, cycloalkanes, alkenes, alkynes, and aromatic hydrocarbons based on functional groups, chain length, and structural features.