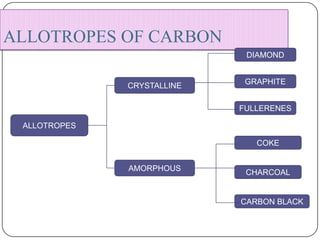
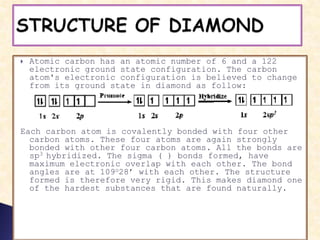
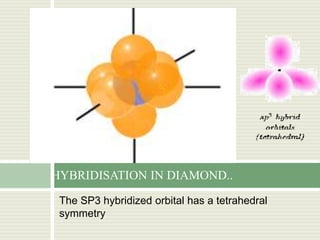
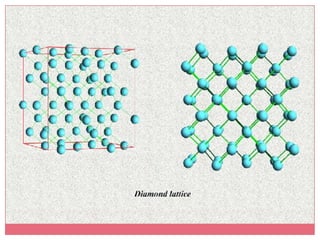
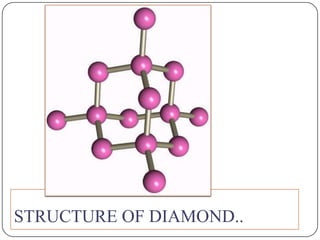
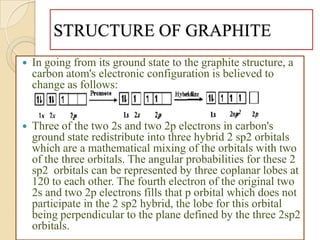
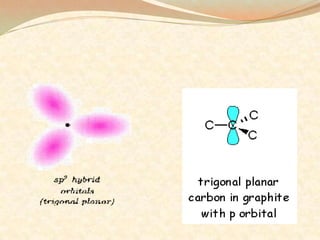
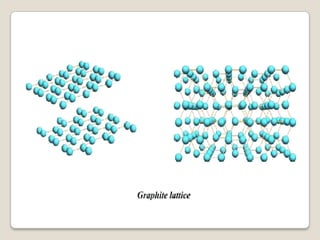
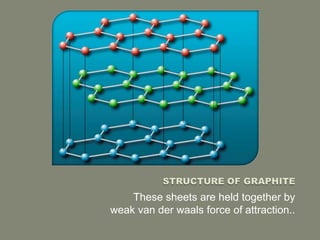
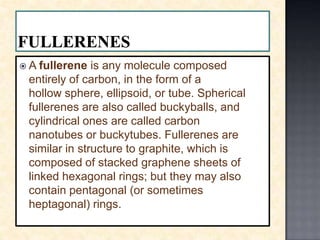
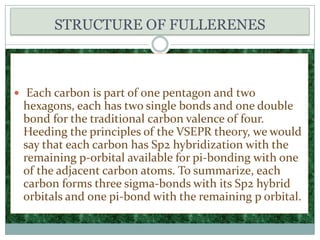
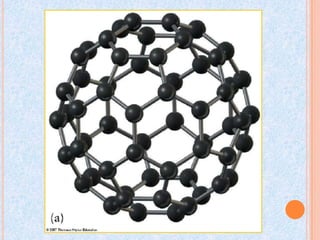
Carbon exists in several allotropes with different structures and properties. Diamond has a tetrahedral structure where each carbon atom is bonded to four other carbons in a rigid three-dimensional lattice. Graphite has stacked sheets of hexagonal rings where each carbon is sp2 hybridized and bonded to three others in each plane, with the planes held weakly together. Fullerene molecules are hollow spheres, tubes, or ellipsoids composed entirely of carbon, with some rings containing five or seven carbons along with the normal six-carbon hexagonal rings.