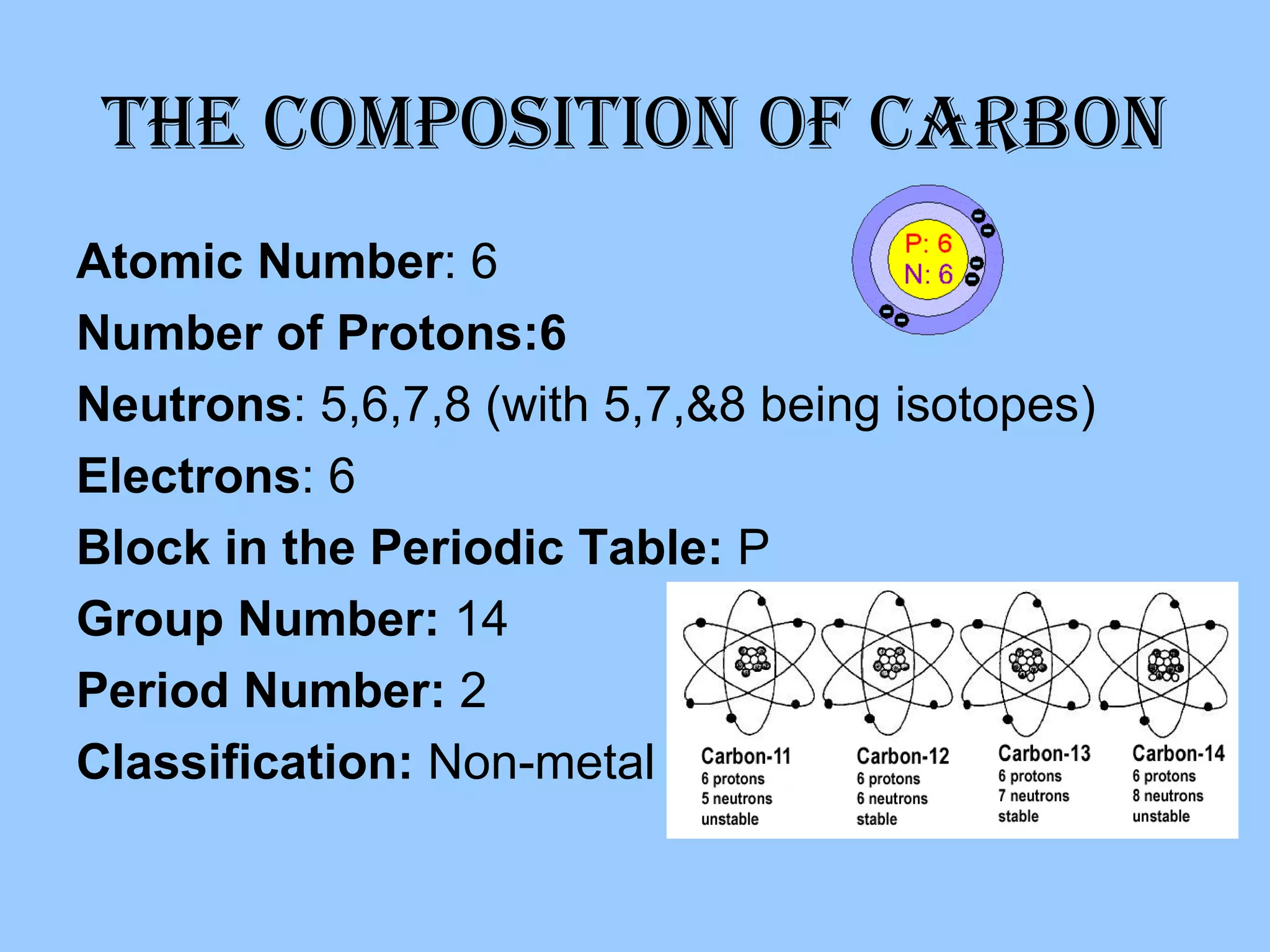
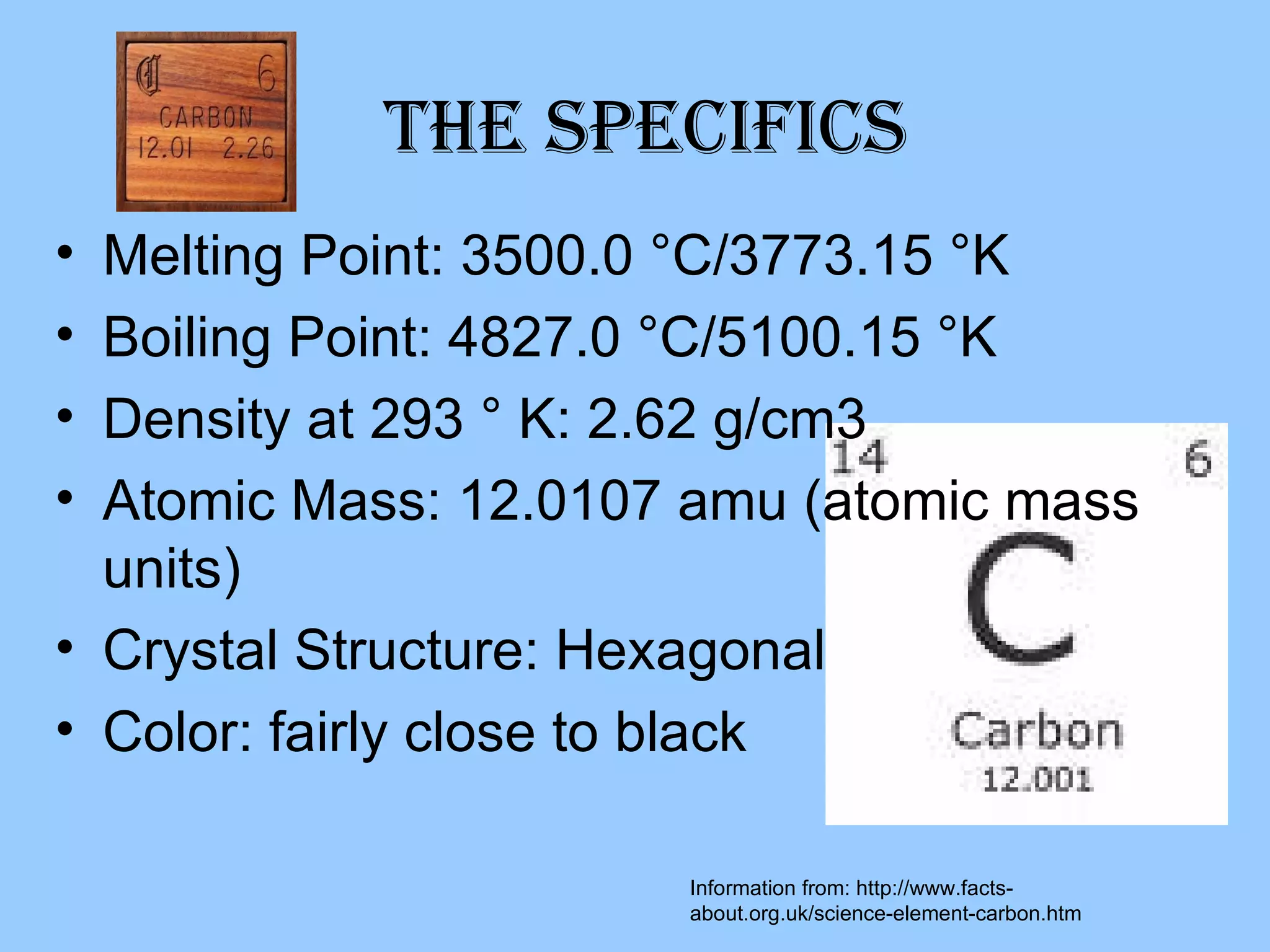
Carbon is the sixth most abundant element in the atmosphere and fourth most in the universe. It is a non-metal found in coal, diamonds, and as the base for organic chemistry and hydrocarbons like fossil fuels. Carbon can be found in its pure form as graphite or diamond, and is known for its use in steel production and as a cooking fuel.