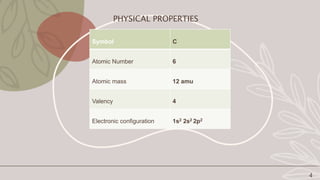
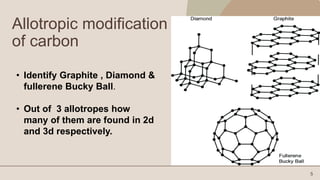
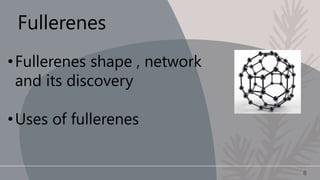
Carbon is a non-metallic element that is essential for all organic life. It has an atomic number of 6 and exists in several allotropes including graphite, diamond, and buckyballs. Carbon is the backbone of organic molecules like DNA, proteins, and carbohydrates. It also plays a critical role in the carbon cycle by which carbon is exchanged between living organisms. The document then discusses the physical and chemical properties of carbon and its allotropes in more detail.





![• Which is the second hardest substance ever found?
I .Sodium peroxoborate
ii .Borazon
iii .Uranium
iv.Na2[B2(O2)2(OH)4].6H2O
• Who discovered graphite?
I. Harold W.Kroto
ii. E .Smalley
iii. Albert Einstein
iv .Both i and ii
• Thermal conductivity of diamond is ……..times more than that of copper
I.3
ii.4
iii.5
iv.6
10](https://image.slidesharecdn.com/hello-bachho-230404135906-85a07000/85/Hello-bachho-pptx-10-320.jpg)
