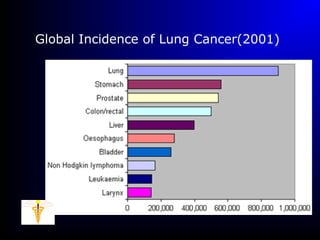
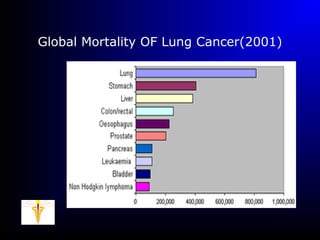
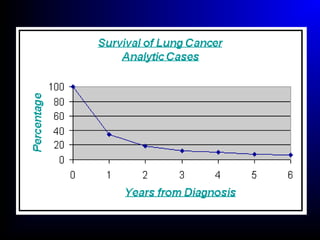
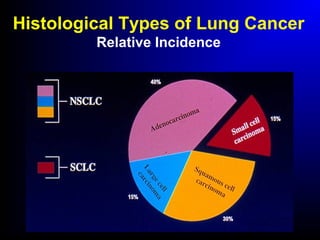
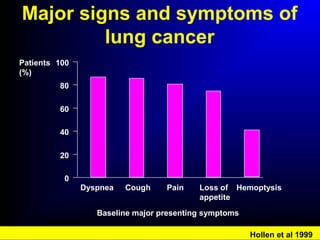
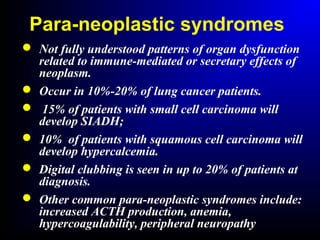
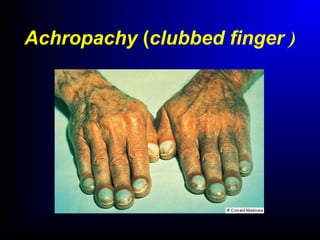
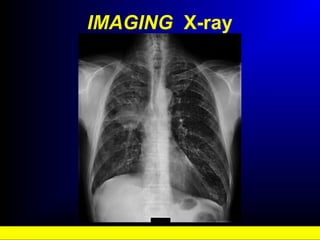
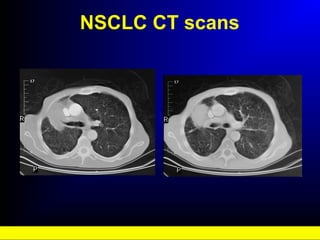
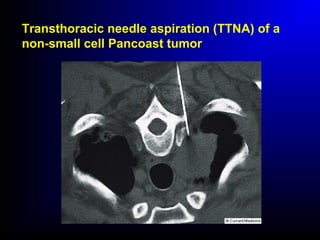
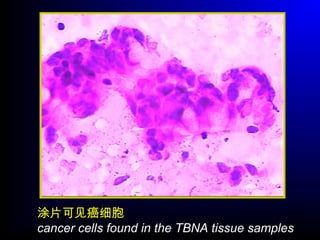
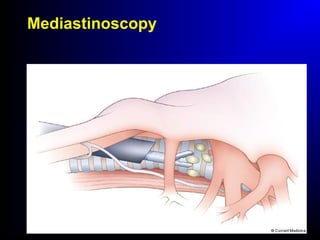
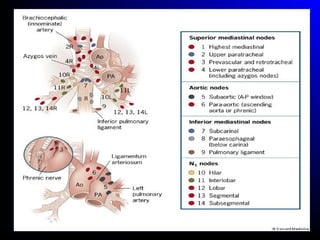
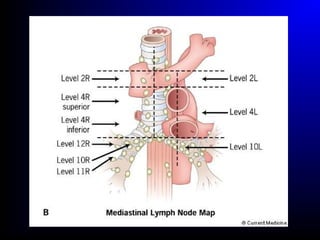
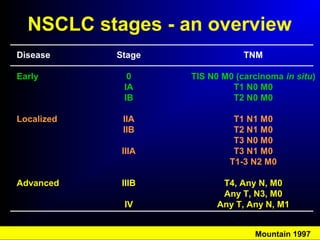
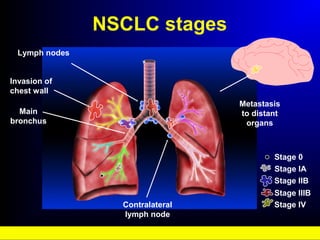
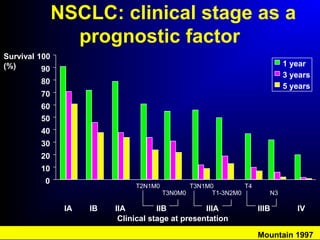
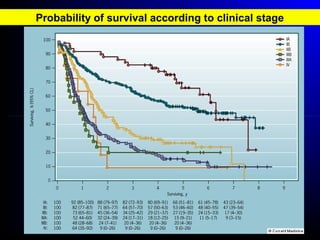
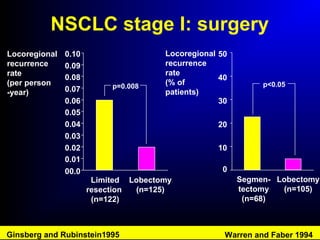
Lung cancer, also known as bronchogenic carcinoma, is a malignant tumor that originates in the lungs. It is the leading cause of cancer death worldwide. The main types are small cell lung cancer and non-small cell lung cancer. Symptoms may include cough, hemoptysis, dyspnea, and weight loss. Diagnosis involves imaging tests and biopsy. Treatment depends on the cancer type and stage, and may include surgery, chemotherapy, radiation therapy, or targeted therapy. Future areas of research focus on earlier detection, improved treatments, and prevention through reducing environmental carcinogens like tobacco smoke.