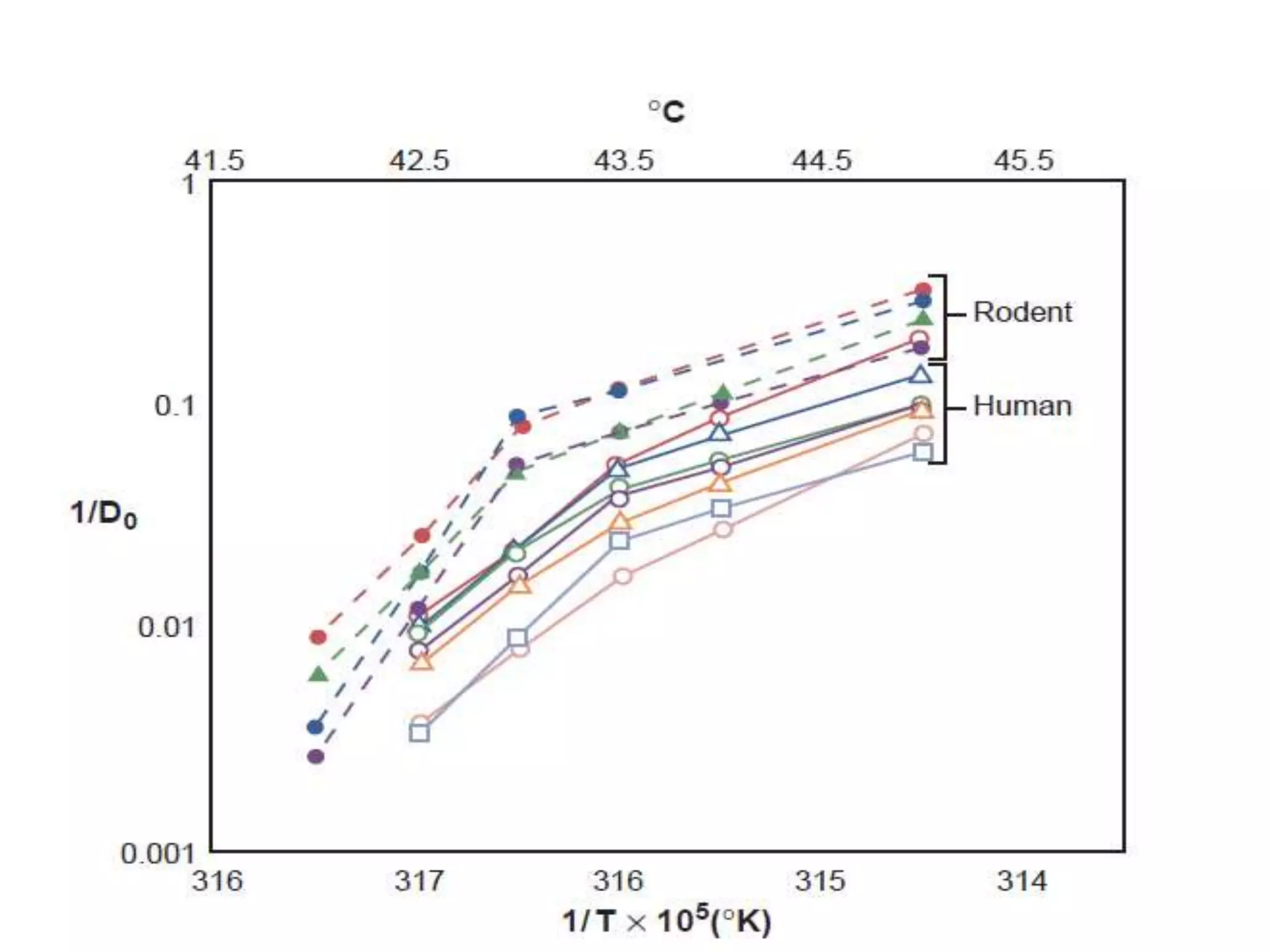
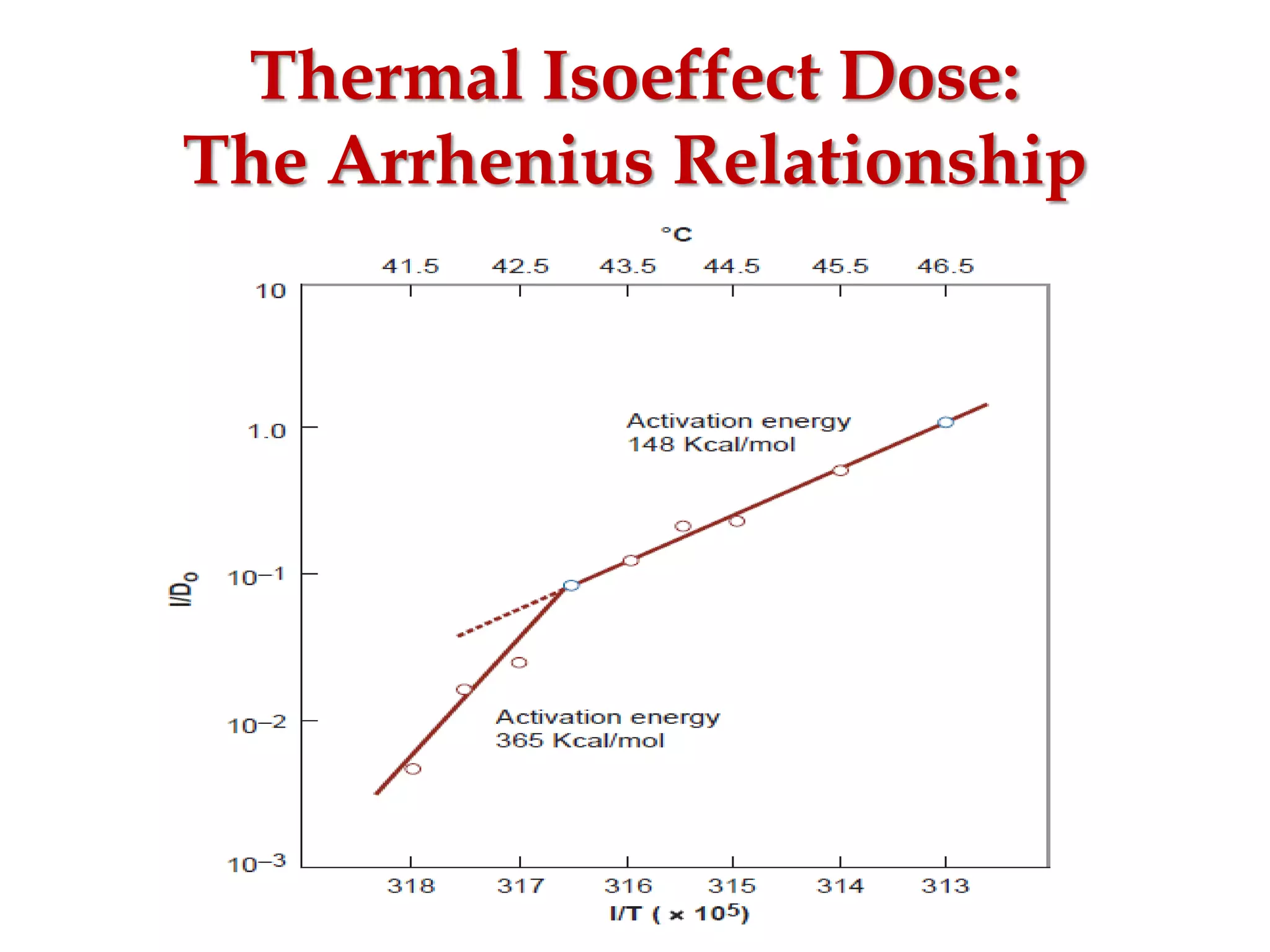
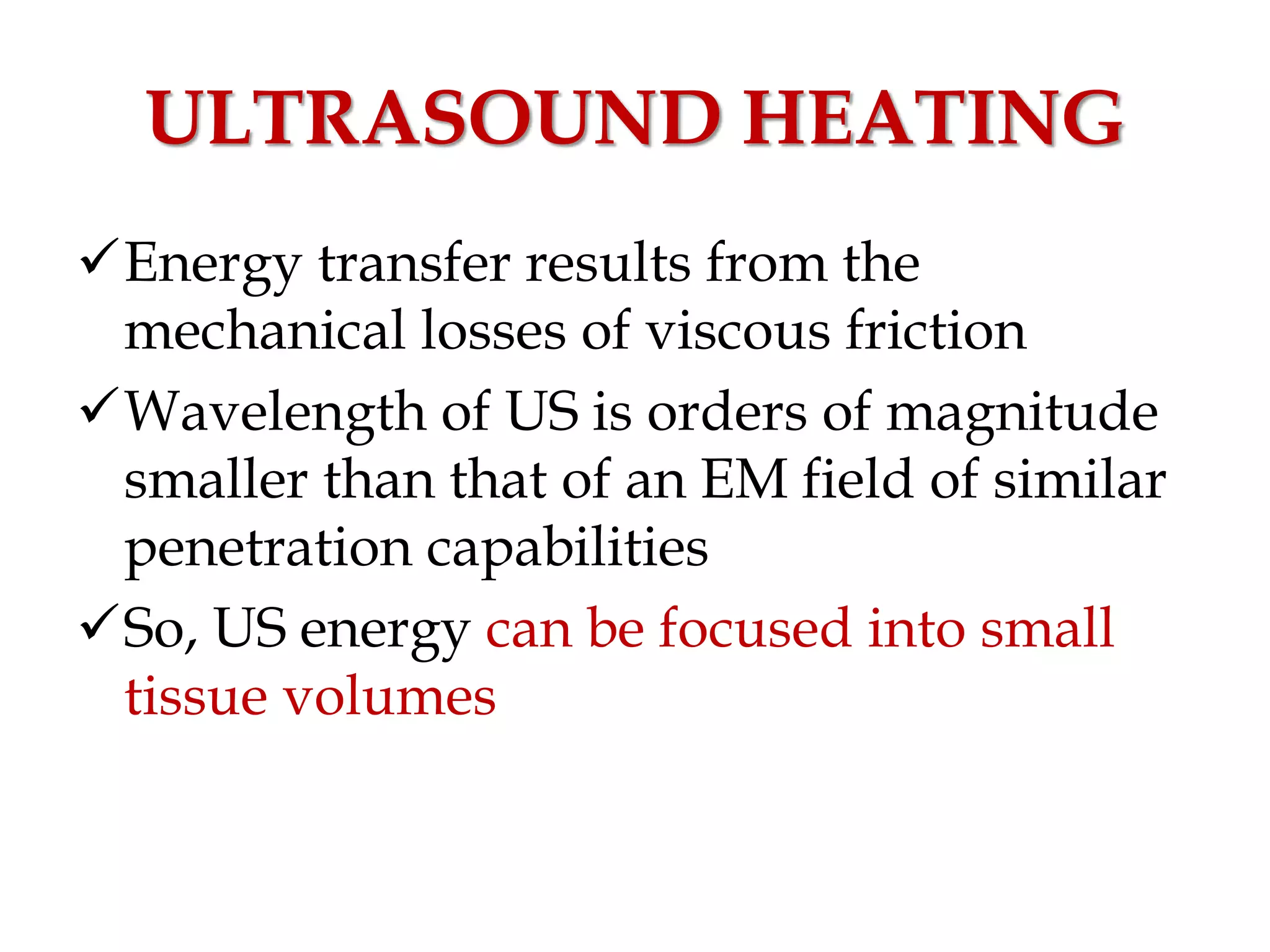
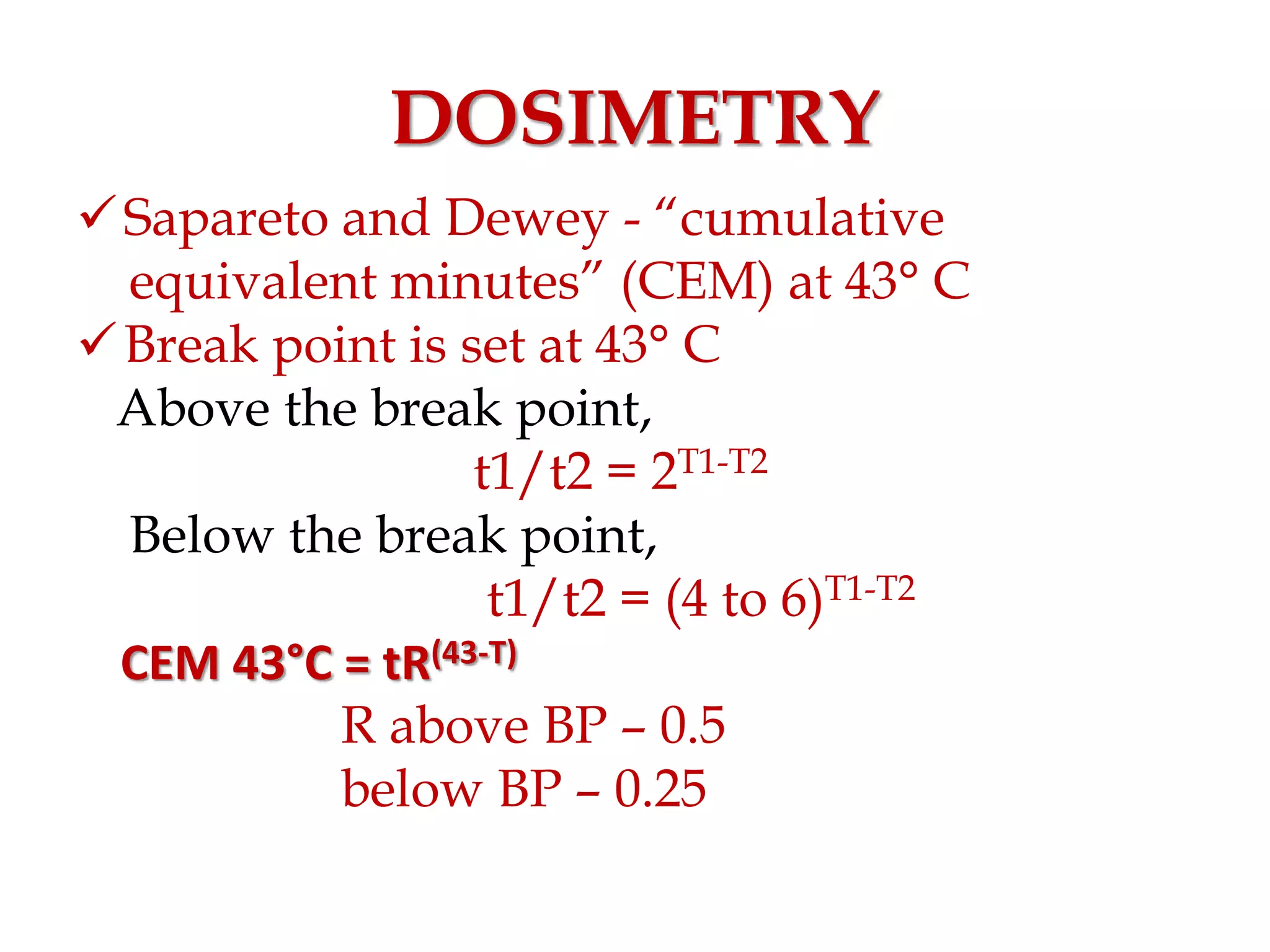
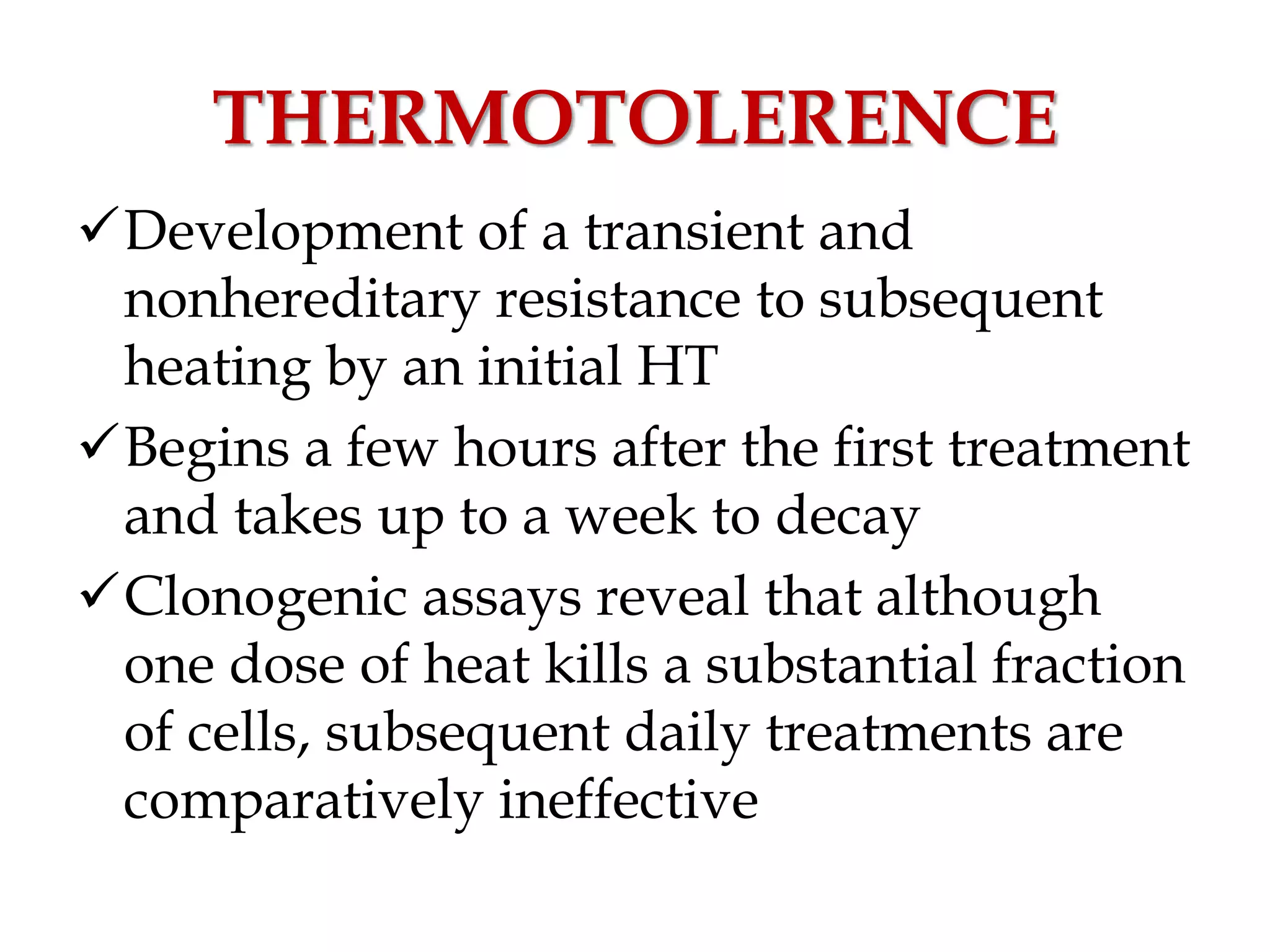
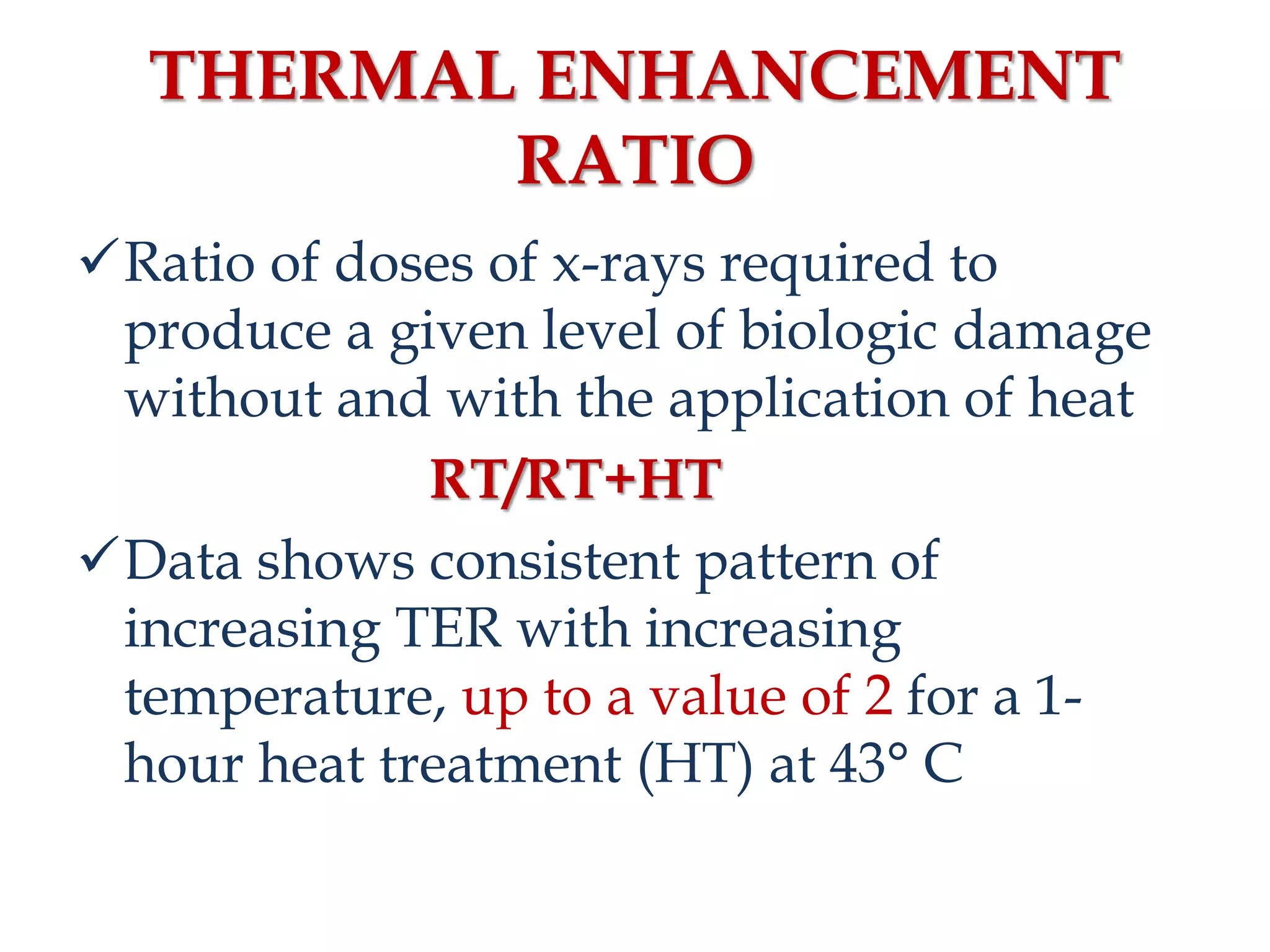
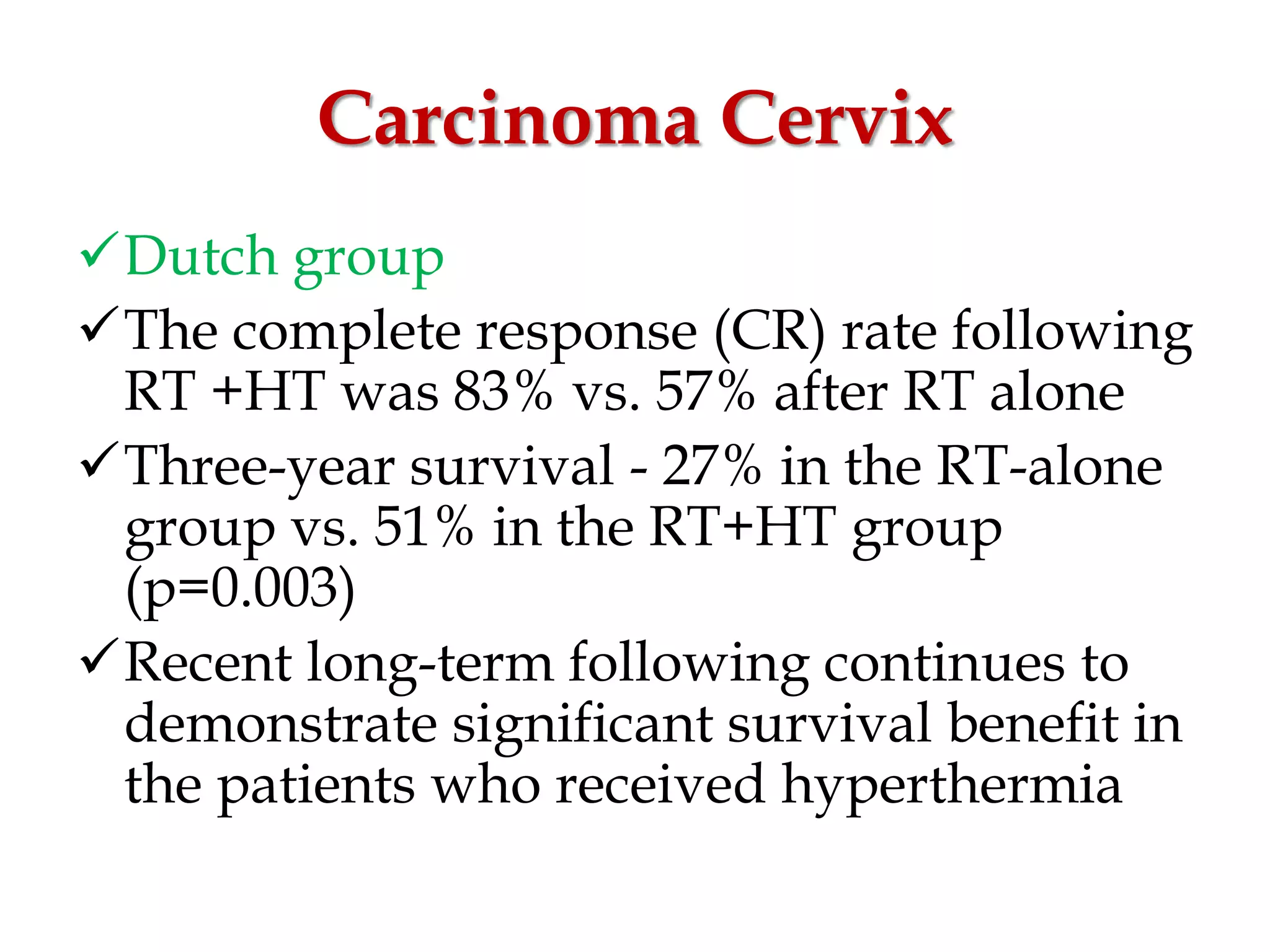
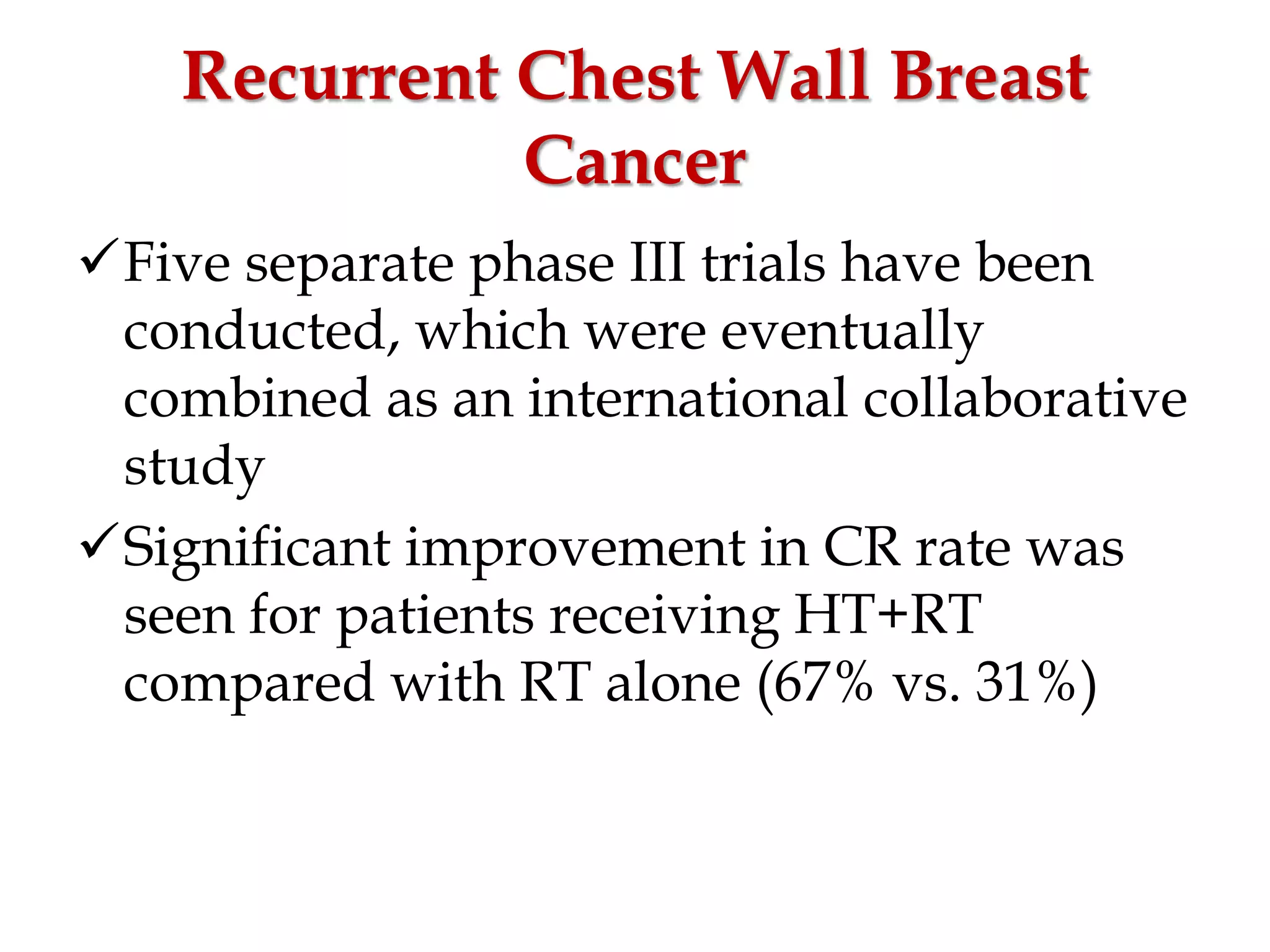
This document discusses hyperthermia in radiotherapy. It provides an overview of the history and biology of hyperthermia, including direct and indirect effects on cells. Temperatures between 41-44°C are used, depending on the region. Hyperthermia enhances radiation therapy by sensitizing cells to radiation, improving oxygenation, and inhibiting DNA repair. Phase III clinical trials have demonstrated improved outcomes when hyperthermia is combined with radiation therapy for various cancers. Challenges include achieving uniform heating and standardizing equipment and dosimetry.