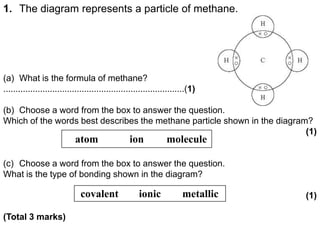
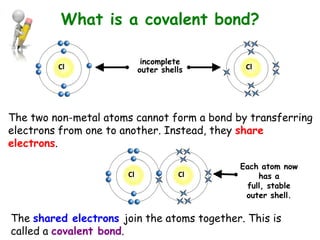
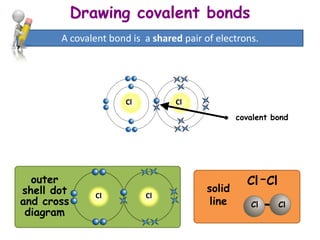
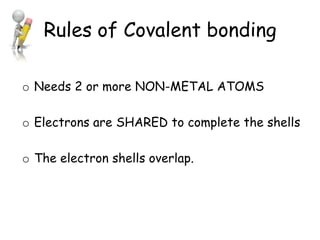
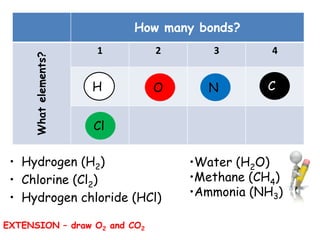
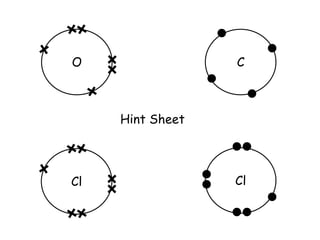
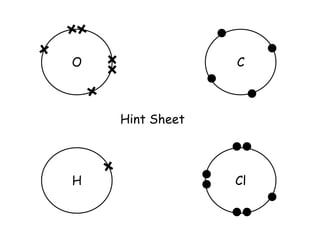
Covalent bonding involves the sharing of electrons between non-metal atoms to achieve stable outer electron shells. A covalent bond is represented by a line or dot-cross diagram that shows the shared pair of electrons joining the atoms. Students learn to identify covalent compounds and draw Lewis dot-cross diagrams to represent covalent bonds between different elements including hydrogen, oxygen, nitrogen, and carbon.