This document discusses isomerism in coordination complexes. It begins with an introduction defining isomerism as compounds with the same chemical formula but different structures. It then describes four main types of isomerism in coordination complexes:
1) Structural isomerism which involves different arrangements of atoms or groups.
2) Ionization isomerism which involves complexes that give different ions in solution.
3) Hydrate isomerism which involves the replacement of coordinated groups by solvent molecules like water.
4) Ligand and linkage isomerism which involve isomers resulting from differences in the ligands themselves or the connectivity of ambidentate ligands. Examples are provided for each type of isomerism.





![ B)Hydrate Isomerism:A very similar type of isomerism results from replacement
of a coordinated group by a solvent molecule (Solvate Isomerism), which in the
case of water is called Hydrate Isomerism. The best known example of this
occurs for chromium chloride (CrCl3⋅6H2O) which may contain 4, 5, or 6
coordinated water molecules (assuming a coordination number of 6). The dot
here is used essentially as an expression of ignorance to indicate that, though
the parts of the molecule separated by the dot are bonded to one another in
some fashion, the exact structural details of that interaction are not fully
expressed in the resulting formula. Using Alfred Werner’s coordination theory
that indicates that several of the water molecules are actually bonded directly
(via coordinate covalent bonds) to the central chromium ion. In fact, there are
several possible compounds that use the brackets to signify bonding in the
complex and the the dots to signify "water molecules that are not bound to the
central metal, but are part of the lattice:
[CrCl2(H2O)4]Cl⋅2H2O: bright-green colored
[CrCl(H2O)5]Cl2⋅H2O: grey-green colored
[Cr(H2O)6]Cl3: violet colored](https://image.slidesharecdn.com/inorganicchemisrty-211018105557/85/Inorganic-Chemisrty-6-320.jpg)
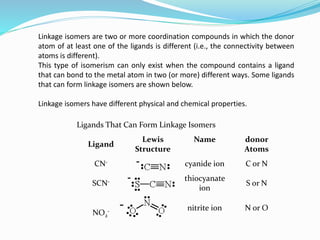
![ D) Linkage Isomerism:Linkage isomerism occurs with ambidentate
ligands. These ligands are capable of coordinating in more than one
way. The best known cases involve the monodentate ligands SCN- /
NCS- and NO2
- / ONO-.
For example: [Co(ONO)(NH3)5] Cl the nitrito isomer -O attached
[Co(NO2)(NH3)5] Cl the nitro isomer - N attached.](https://image.slidesharecdn.com/inorganicchemisrty-211018105557/85/Inorganic-Chemisrty-8-320.jpg)