The document discusses various concepts related to acid-base balance in the body:
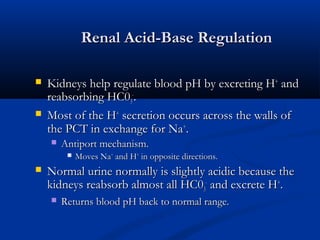
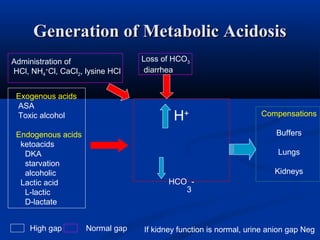
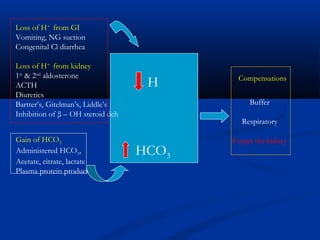
1) It describes the anion gap and how clinicians use it to identify the cause of metabolic acidosis. It also discusses the normal bicarbonate/carbon dioxide buffer system and how it helps regulate pH.
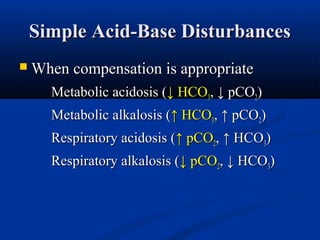
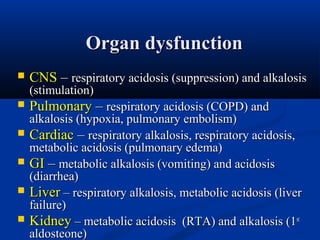
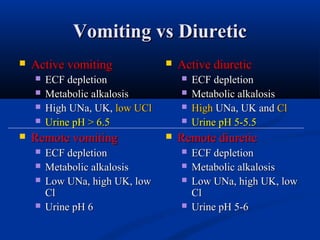
2) Several potential causes of respiratory and metabolic acidosis and alkalosis are outlined, including renal and respiratory mechanisms of compensation.
3) Treatment approaches for different acid-base disturbances are provided, such as sodium bicarbonate administration for metabolic acidosis and rebreathe bags for respiratory alkalosis.
![Anion Gap
The difference between [Na+] and the sum of
[HC03-] and [Cl-].
[Na+] – ([HC0 -] + [Cl-]) =
3
140 - (24 + 105) = 11
Normal = 12 + 2
Clinicians use the anion gap to identify the cause
of metabolic acidosis.](https://image.slidesharecdn.com/acidbasedisturbances-131203055138-phpapp02/85/Acid-base-disturbances-1-320.jpg)

![Buffer: any substance which reversibly consumes or releases H+. Buffers minimize or
attenuate changes in pH by consuming or adding H+ in such a way to minimize discrete
changes.
Valence does not matter, ie for buffer “B”
Protonated form in equilibrium with deporotonated form
Weak acid
HB (n+1)
Weak base
B (n) + H (+)
=
The buffers distribute themselves via their dissociation constant (K) defined as the ratio
[B(n)] [H+] = K
[HB(n=1)]](https://image.slidesharecdn.com/acidbasedisturbances-131203055138-phpapp02/85/Acid-base-disturbances-3-320.jpg)