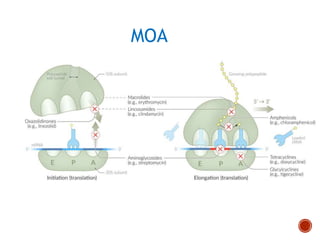
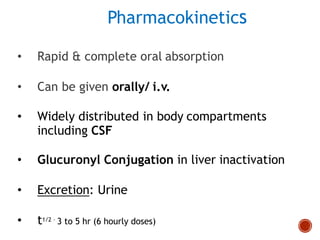
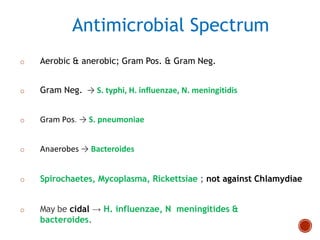
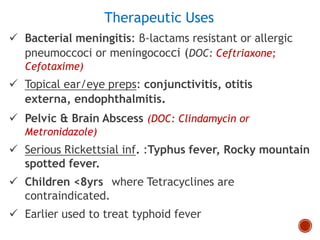
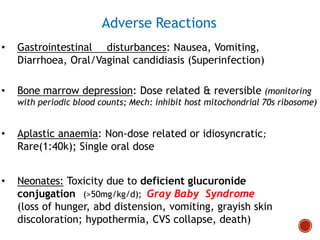
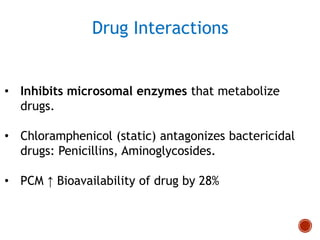
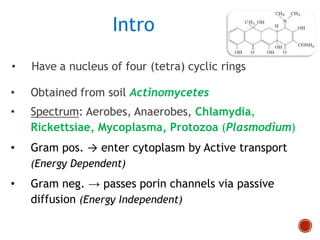
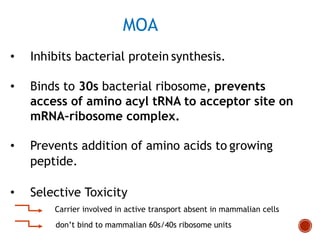
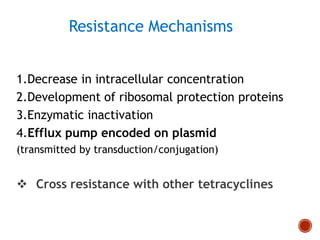
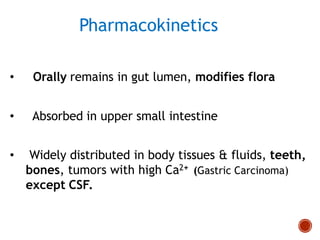
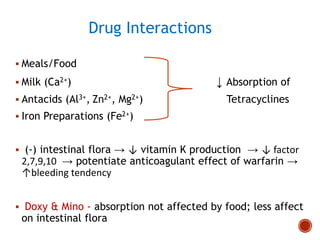
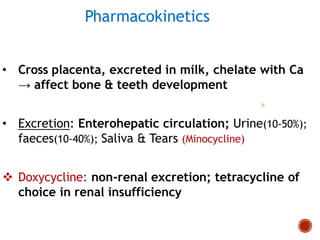
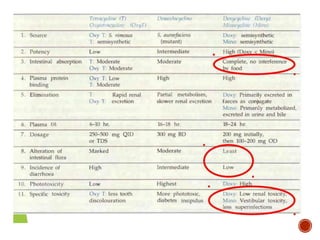
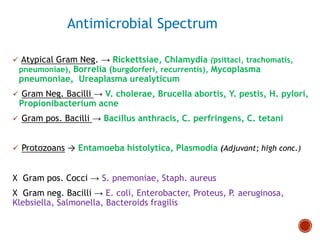
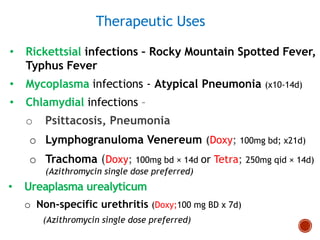
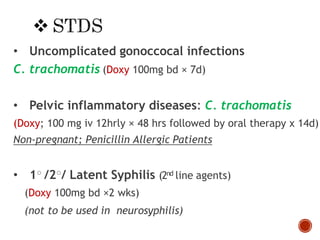
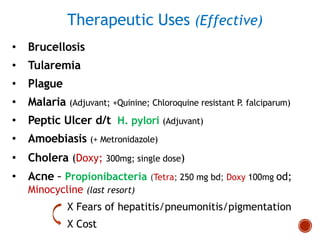
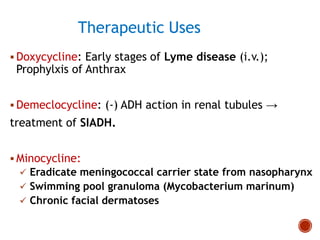
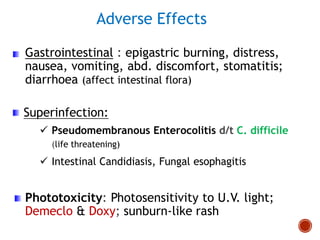
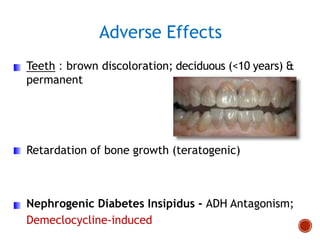
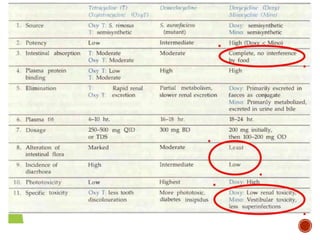
Chloramphenicol and tetracyclines are broad-spectrum antibiotics that are bacteriostatic. Their widespread use led to many resistant bacterial strains. Chloramphenicol works by reversibly binding to the 50S ribosomal subunit and inhibiting peptidyl transferase activity. Tetracyclines inhibit bacterial protein synthesis by binding to the 30S ribosomal subunit. Both experience resistance through ribosomal protection, decreased drug permeability/concentration, and enzymatic inactivation. They have many therapeutic uses but also potential adverse effects like bone/tooth discoloration and gastrointestinal issues. Tigecycline is a newer glycylcycline antibiotic with activity against resistant strains.