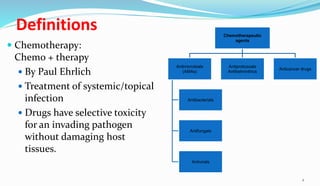
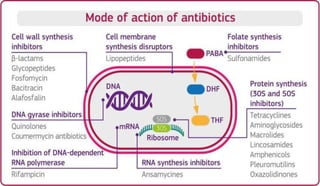
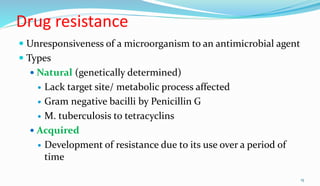
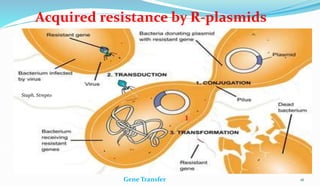
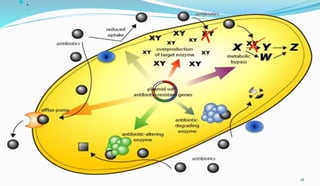
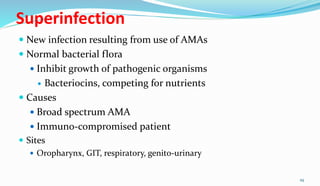
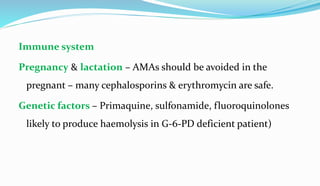
This document provides definitions and information about chemotherapy and antimicrobial agents. It discusses the history of chemotherapy beginning with Paul Ehrlich and the discovery of penicillin. It then classifies antibiotics according to spectrum of activity, type of action, organisms targeted, mechanism of action, chemical structure, and source. The document discusses drug resistance, combination therapy, chemoprophylaxis, superinfection, and principles of rational antibiotic use. It emphasizes the importance of proper antibiotic selection, dosage, duration and monitoring treatment to improve outcomes and reduce drug resistance.