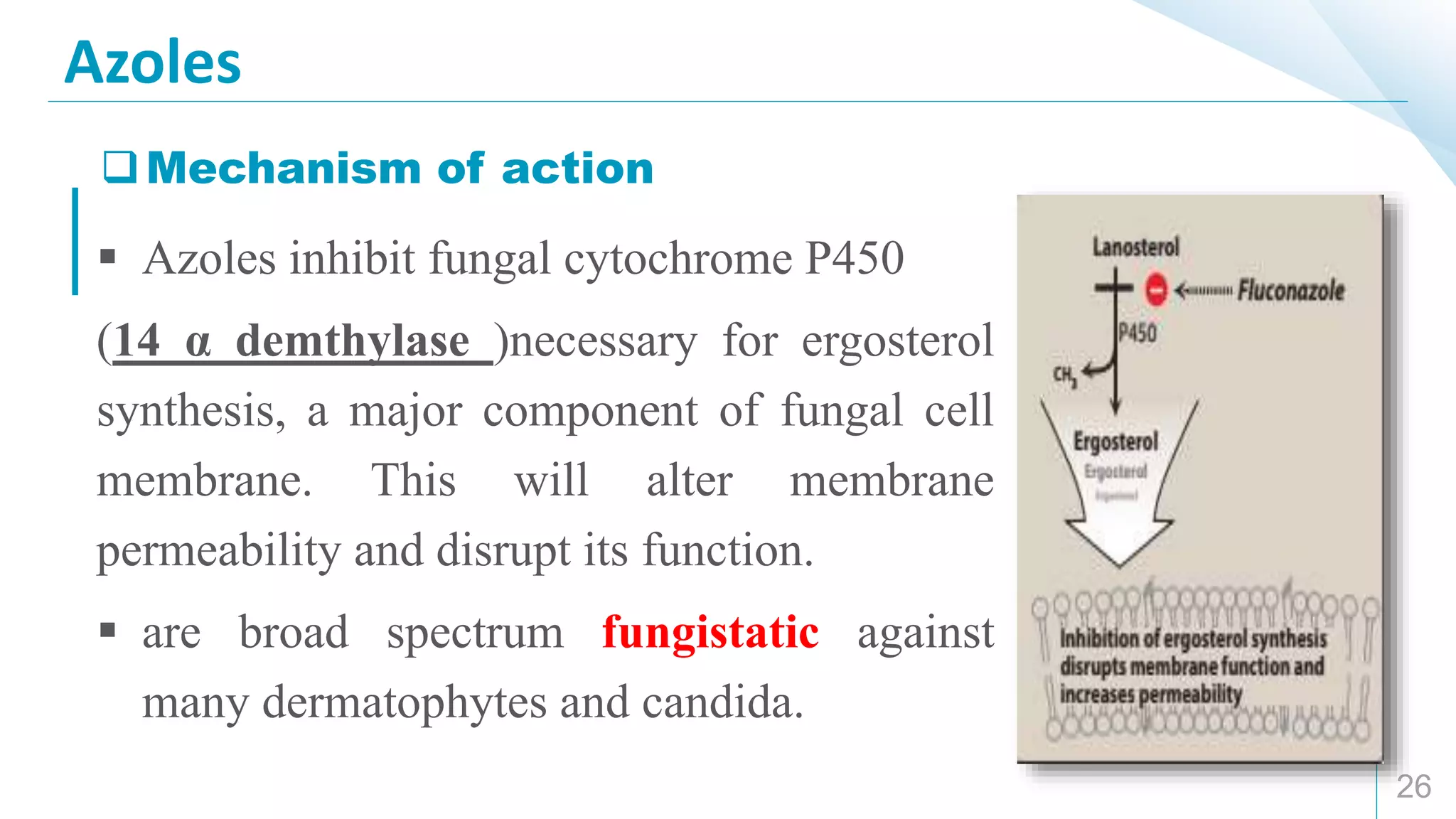
This document provides an overview of anti-fungal drugs. It begins by classifying antifungals based on their chemical structure, sites of action, and mechanisms of action. The major classes discussed include azoles, polyene macrolides, and other antifungals. Azoles like fluconazole and itraconazole are broad-spectrum and inhibit ergosterol synthesis. Amphotericin B binds to ergosterol and forms pores in fungal membranes. Other antifungals discussed are flucytosine, griseofulvin, and nystatin. The document outlines the mechanisms, therapeutic uses, and adverse effects of the main antifungal drug

























![Azoles
28
Therapeutic uses
I. Superficial fungal infections: [ketoconazole – itraconazole –
miconazole]
1. Dermatophytes infection of the skin (tinea), hair, and nails
(onychomycosis):
For skin infection: treatment continued for 2-4 weeks.
For hair infection: treatment continued for 6-8 weeks.
For nail infection: treatment continued for 3-6 months.
2. Mucocautaneous candidiasis: oropharyngeal, vulvovaginal, etc.](https://image.slidesharecdn.com/anti-fungal-180303181604/75/Anti-Fungal-drugs-28-2048.jpg)
![Azoles
29
II. Systemic fungal infections: [itraconazole – fluconazole –
voriconazole]
Itraconazole (orally or IV) is the drug of choice for systemic
blastomycosis.
Fluconazole (orally or IV) is the drug of choice for systemic
candidiasis, and cryptococcal meningitis (because it the only azole
that can cross to CSF with good concentration).
Voriconazole is the drug of choice for inVasive aspergillosis of the
lung.](https://image.slidesharecdn.com/anti-fungal-180303181604/75/Anti-Fungal-drugs-29-2048.jpg)





















