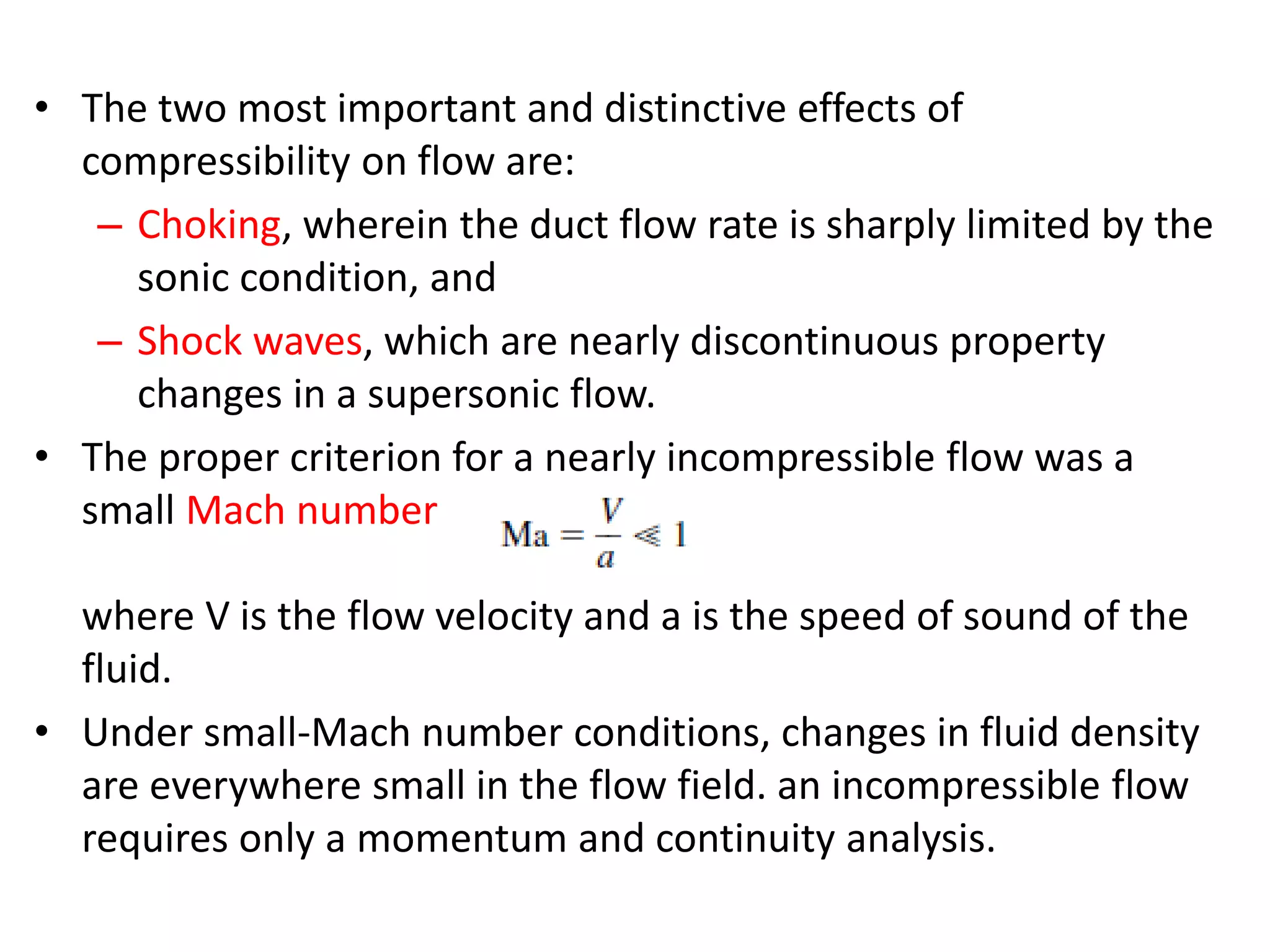
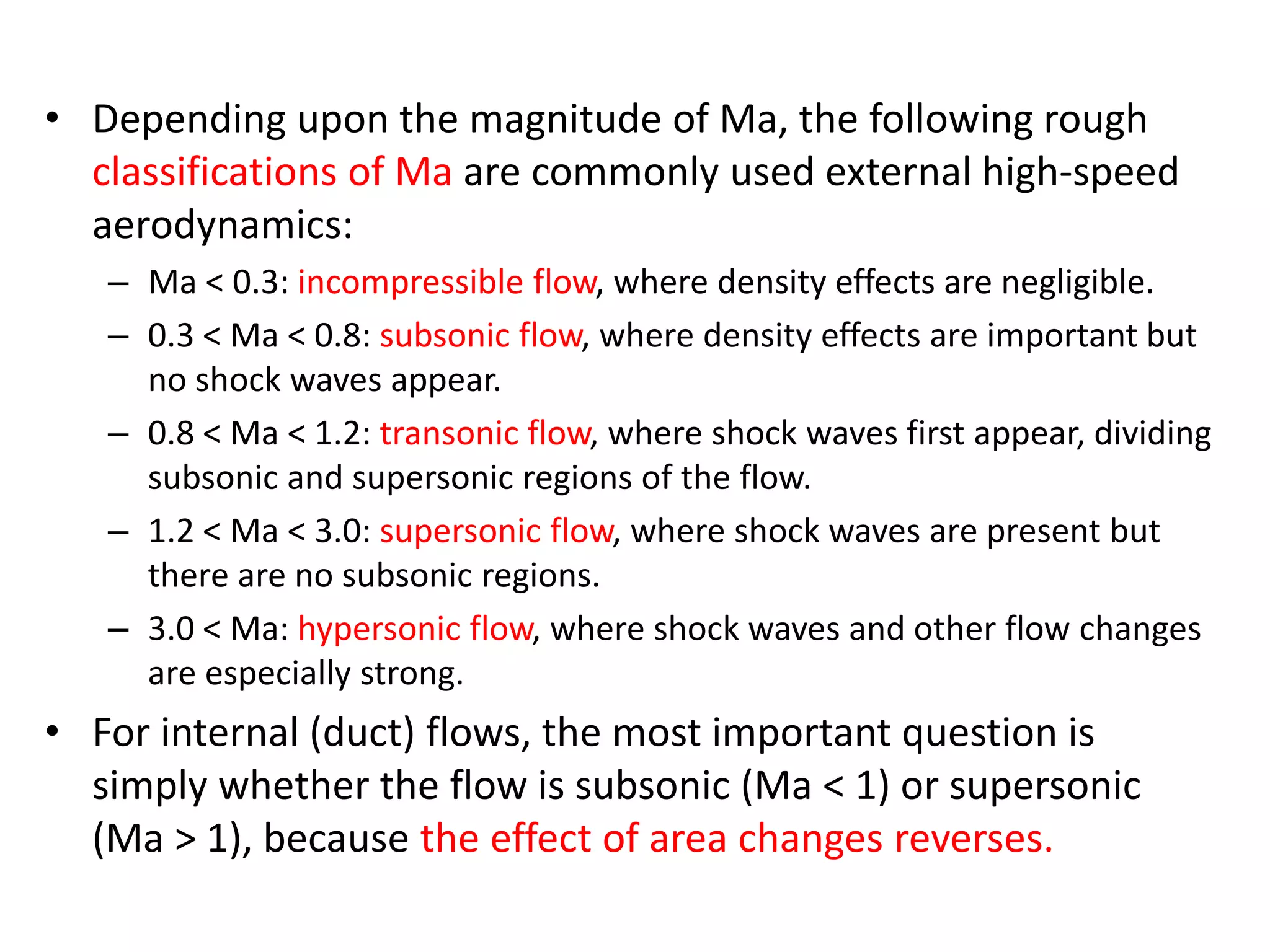
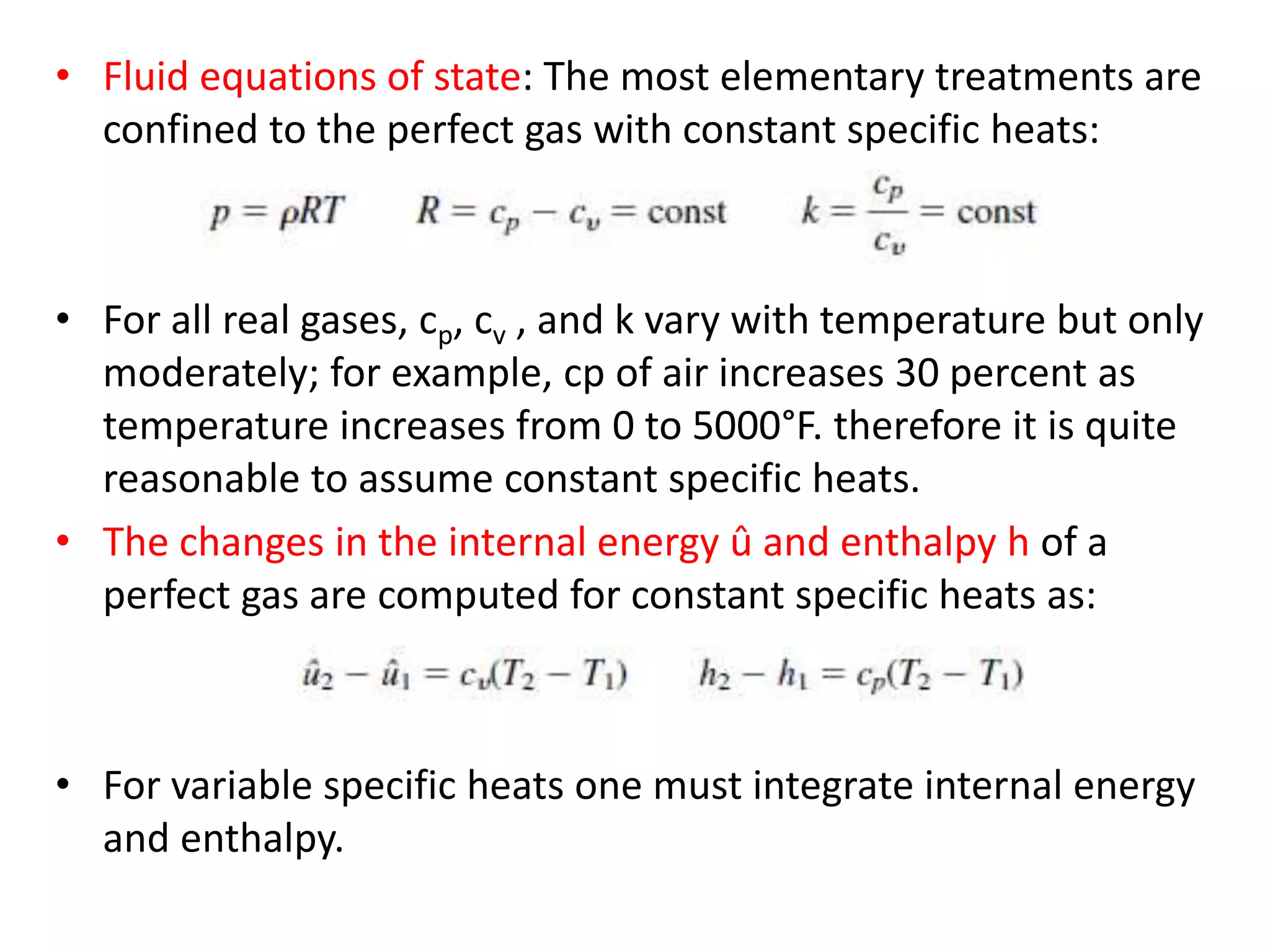
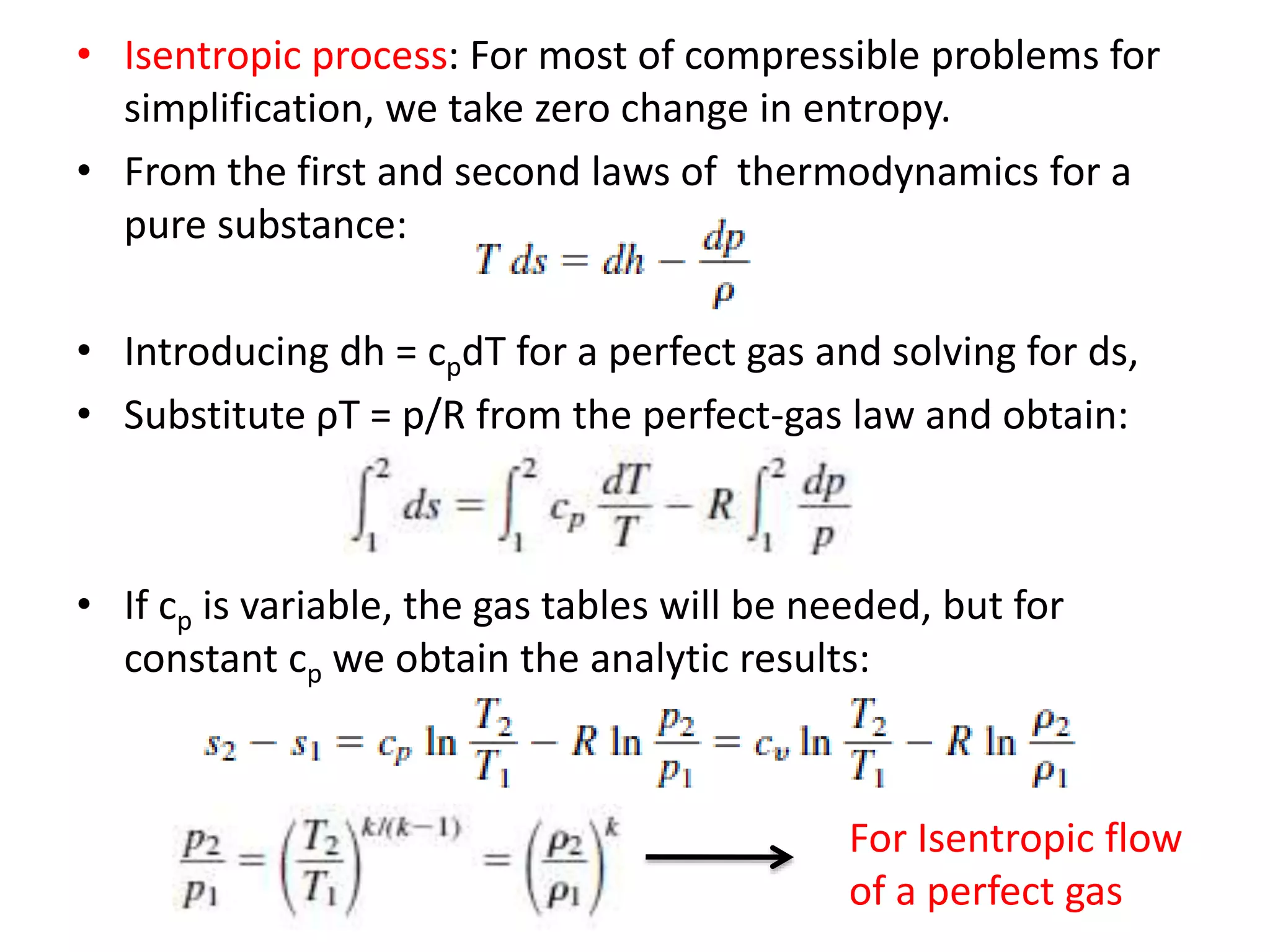
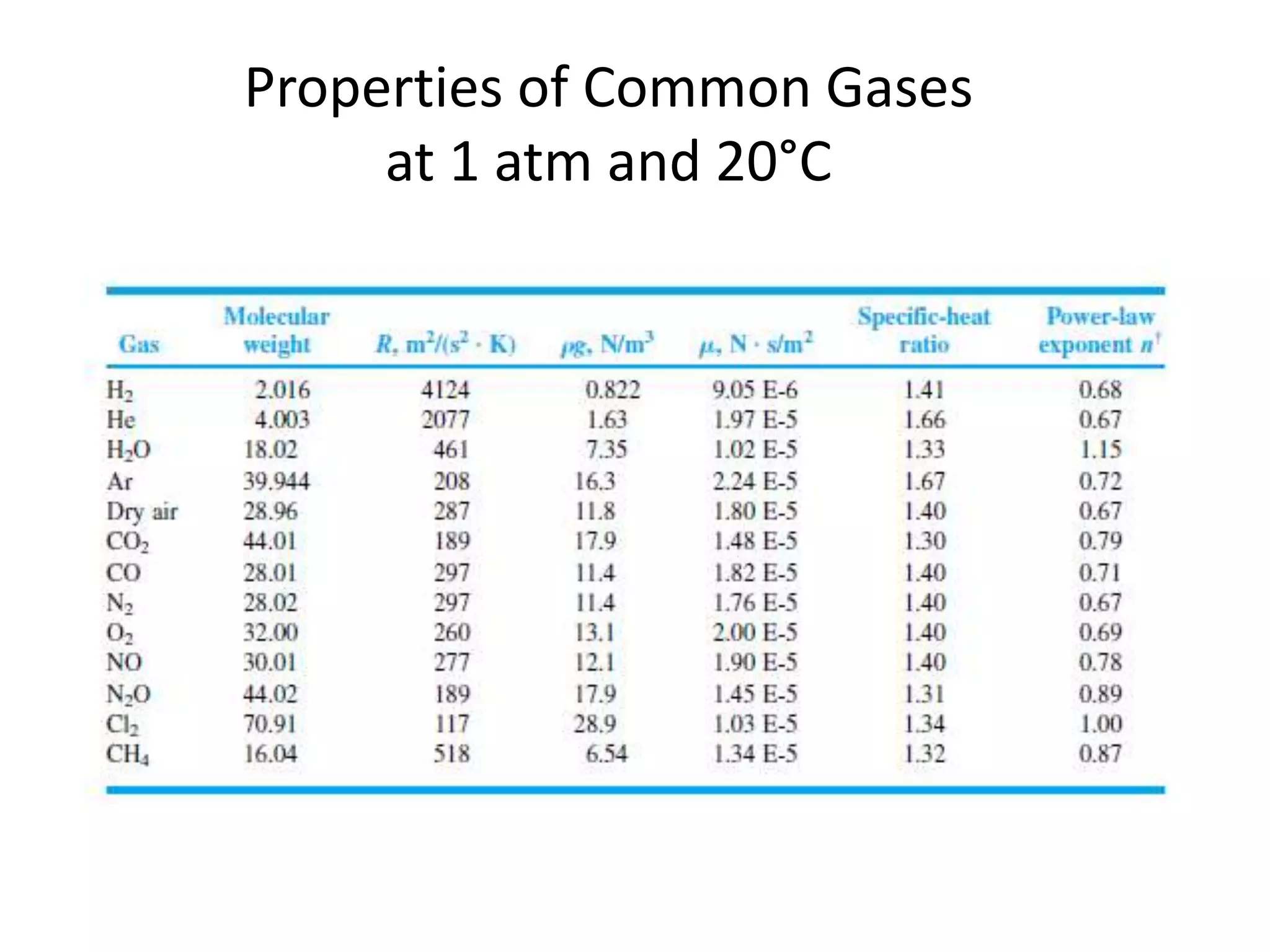
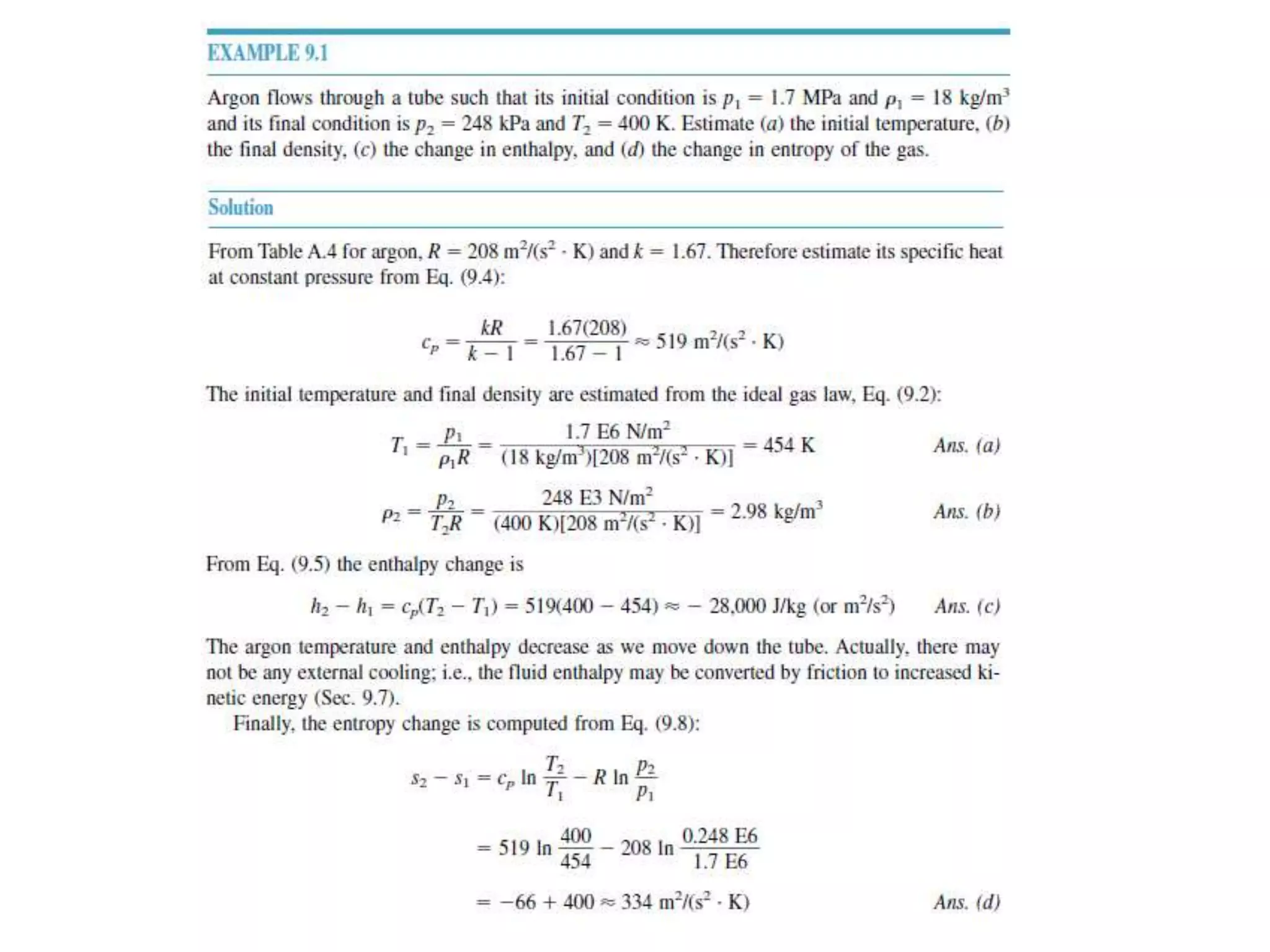
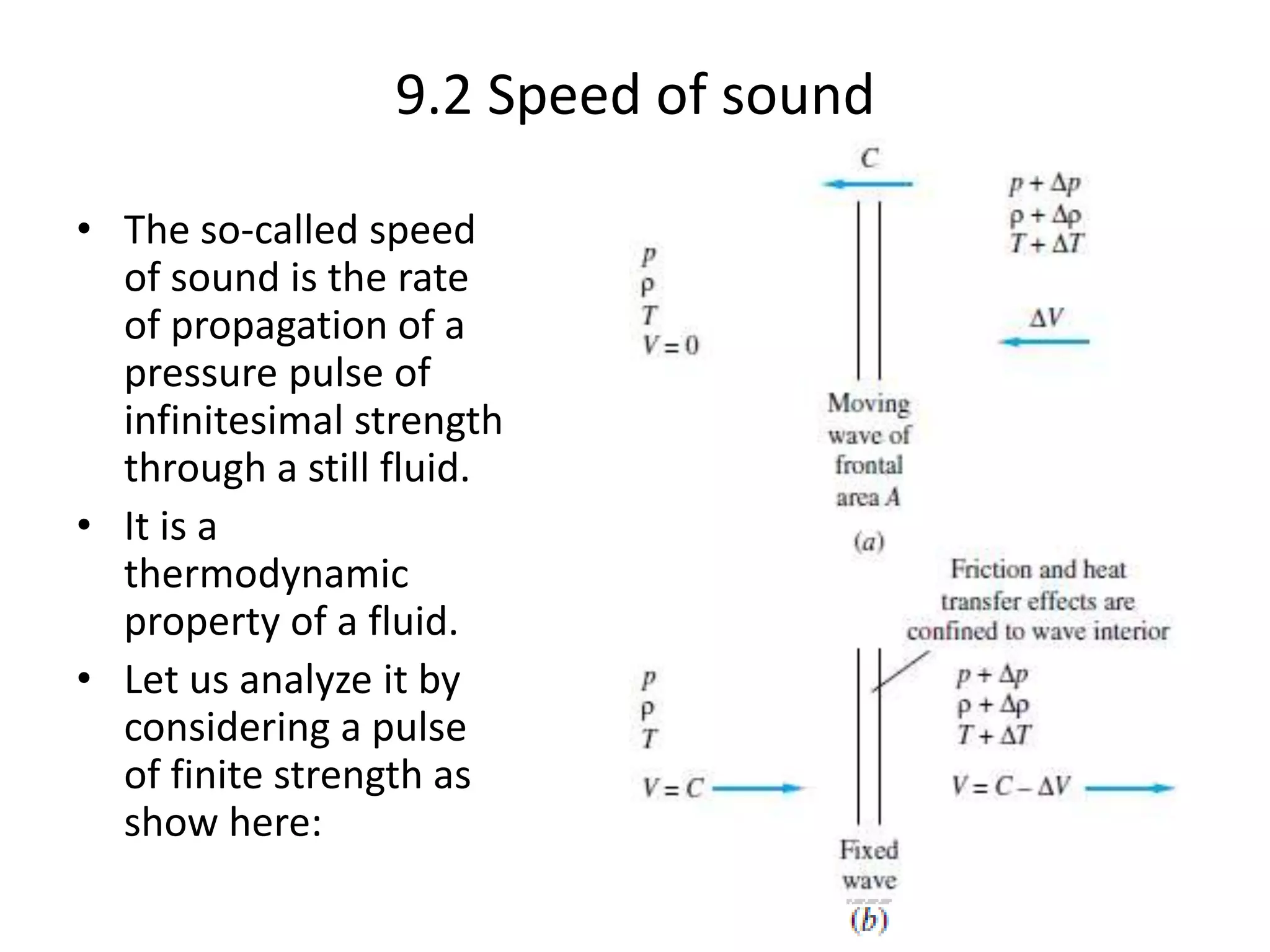
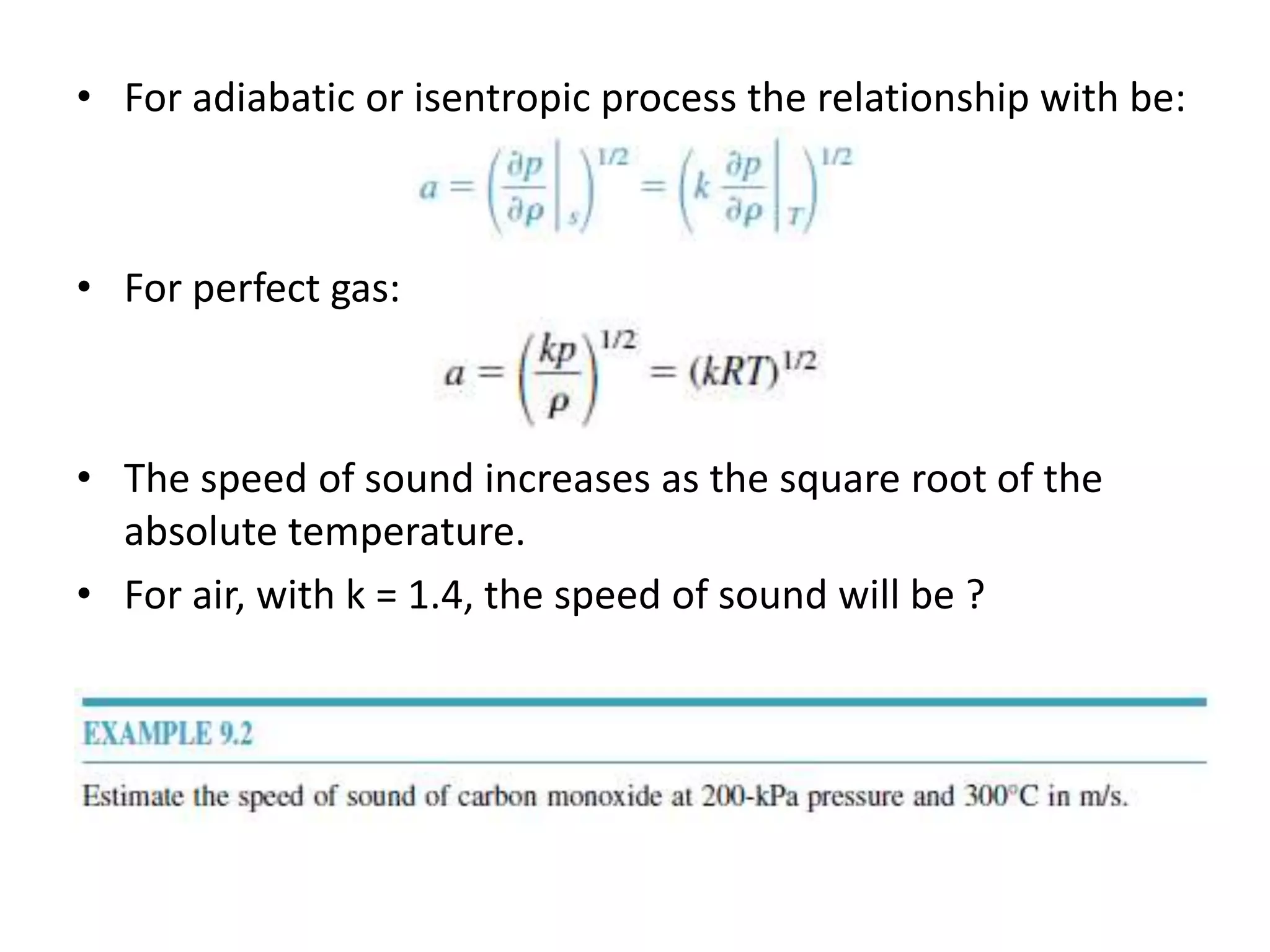
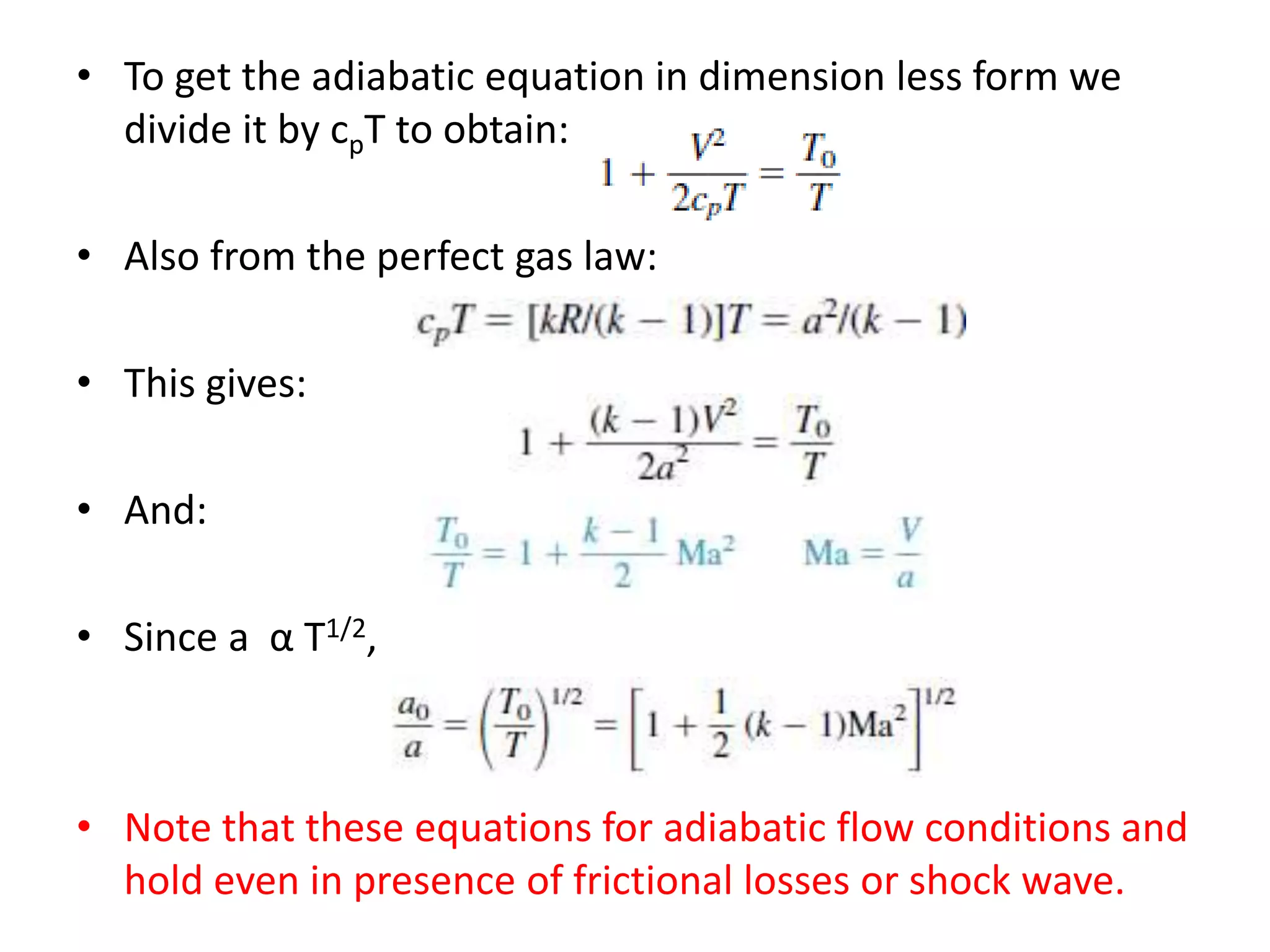
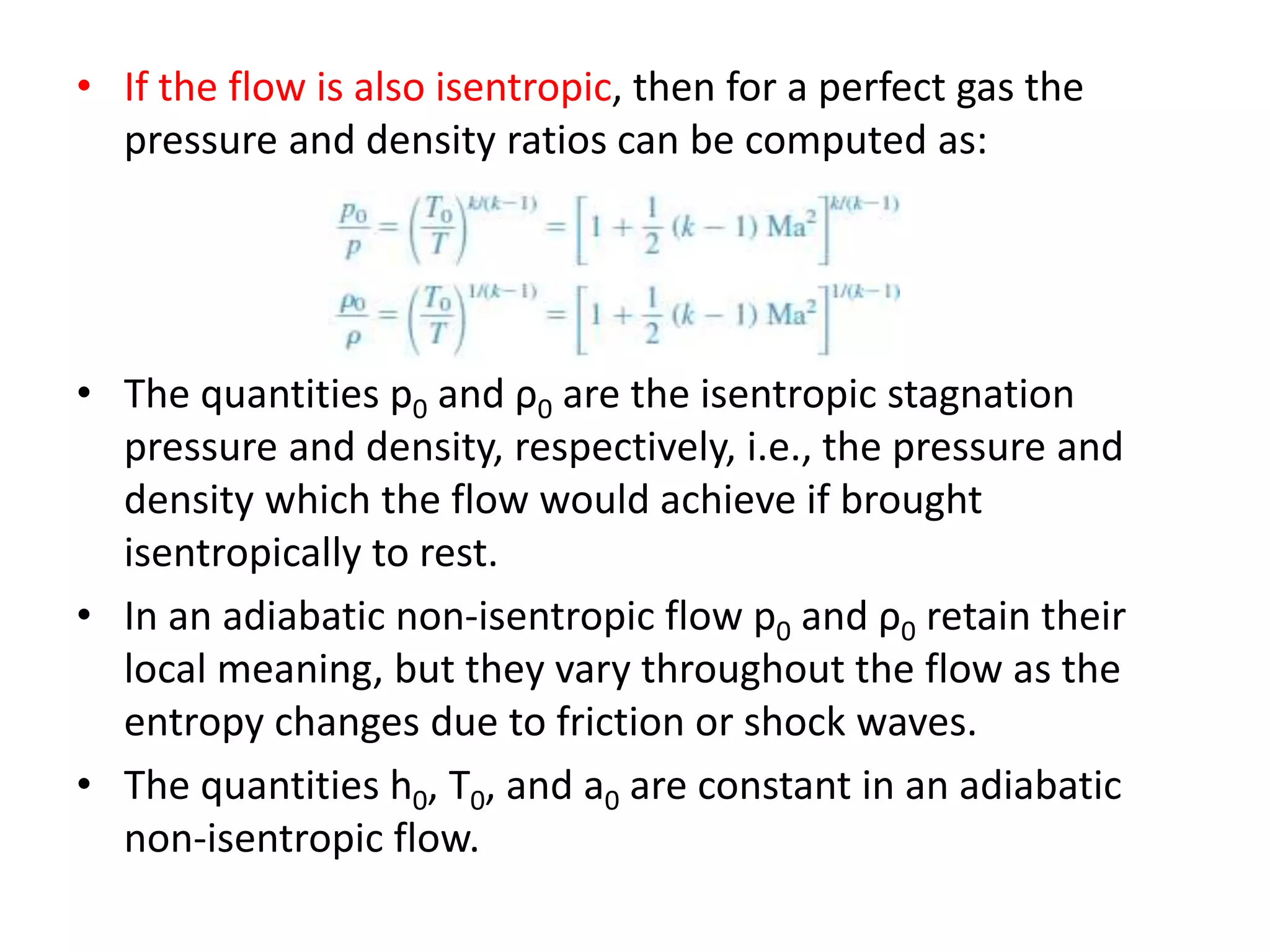
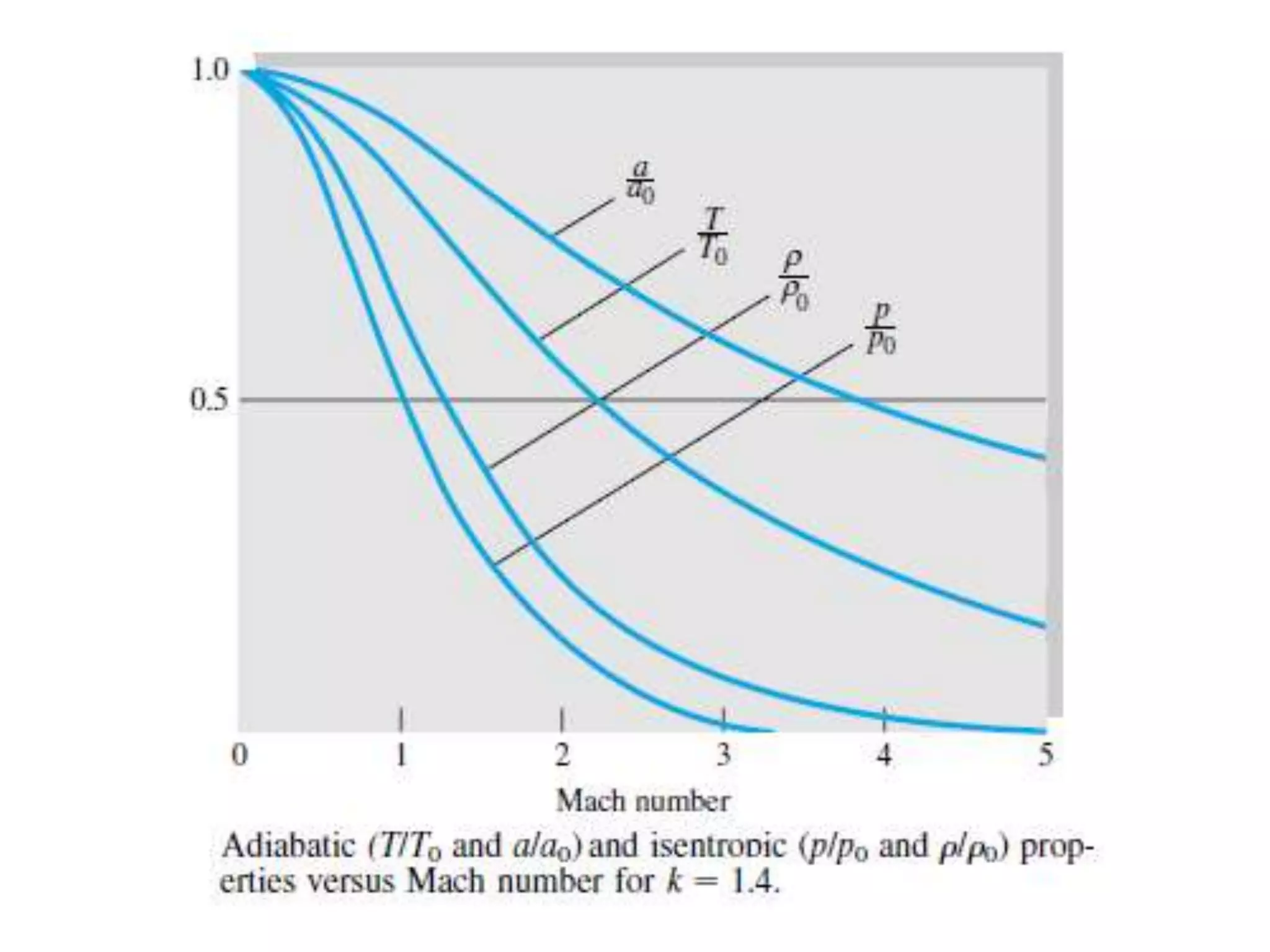
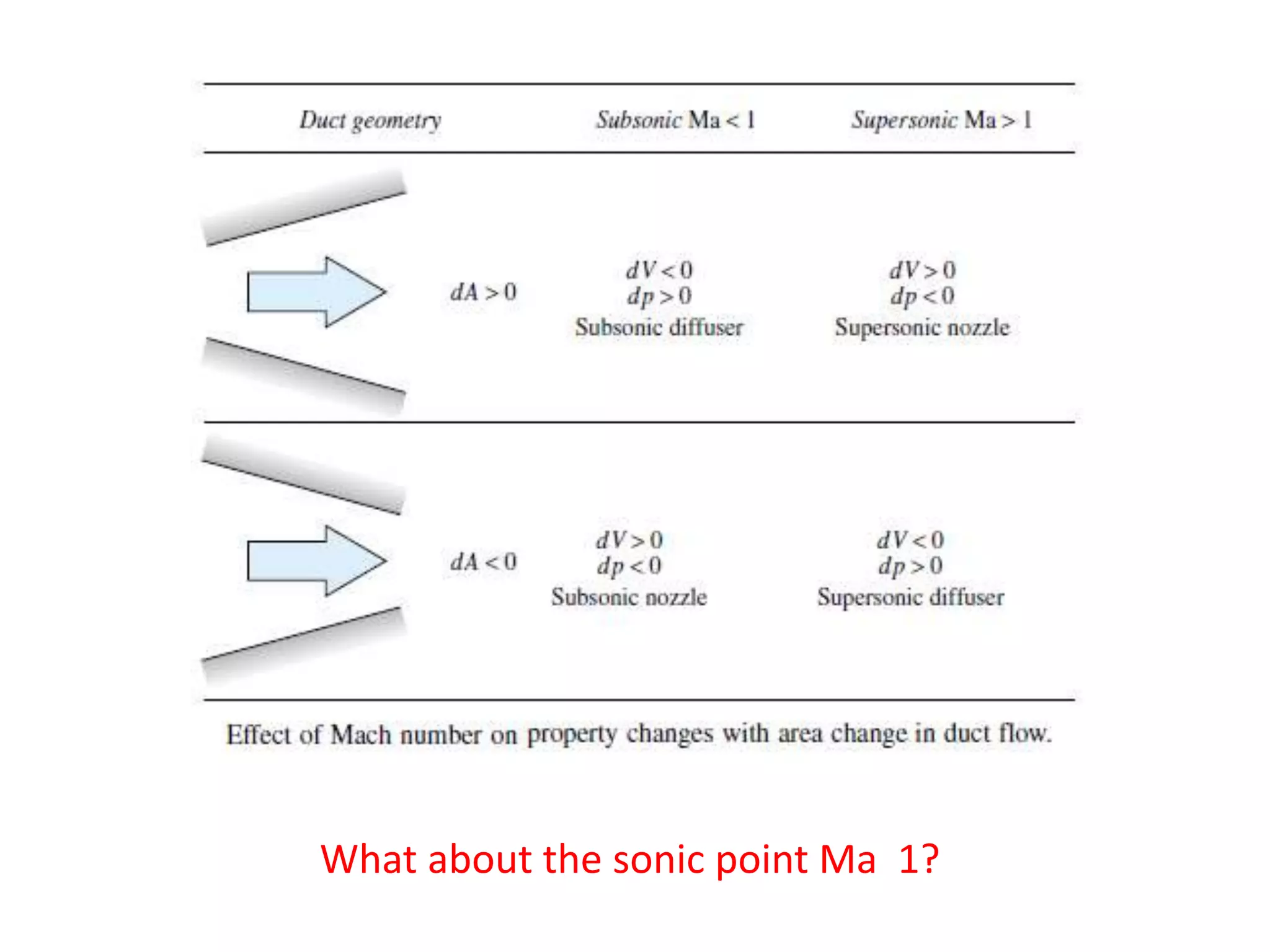
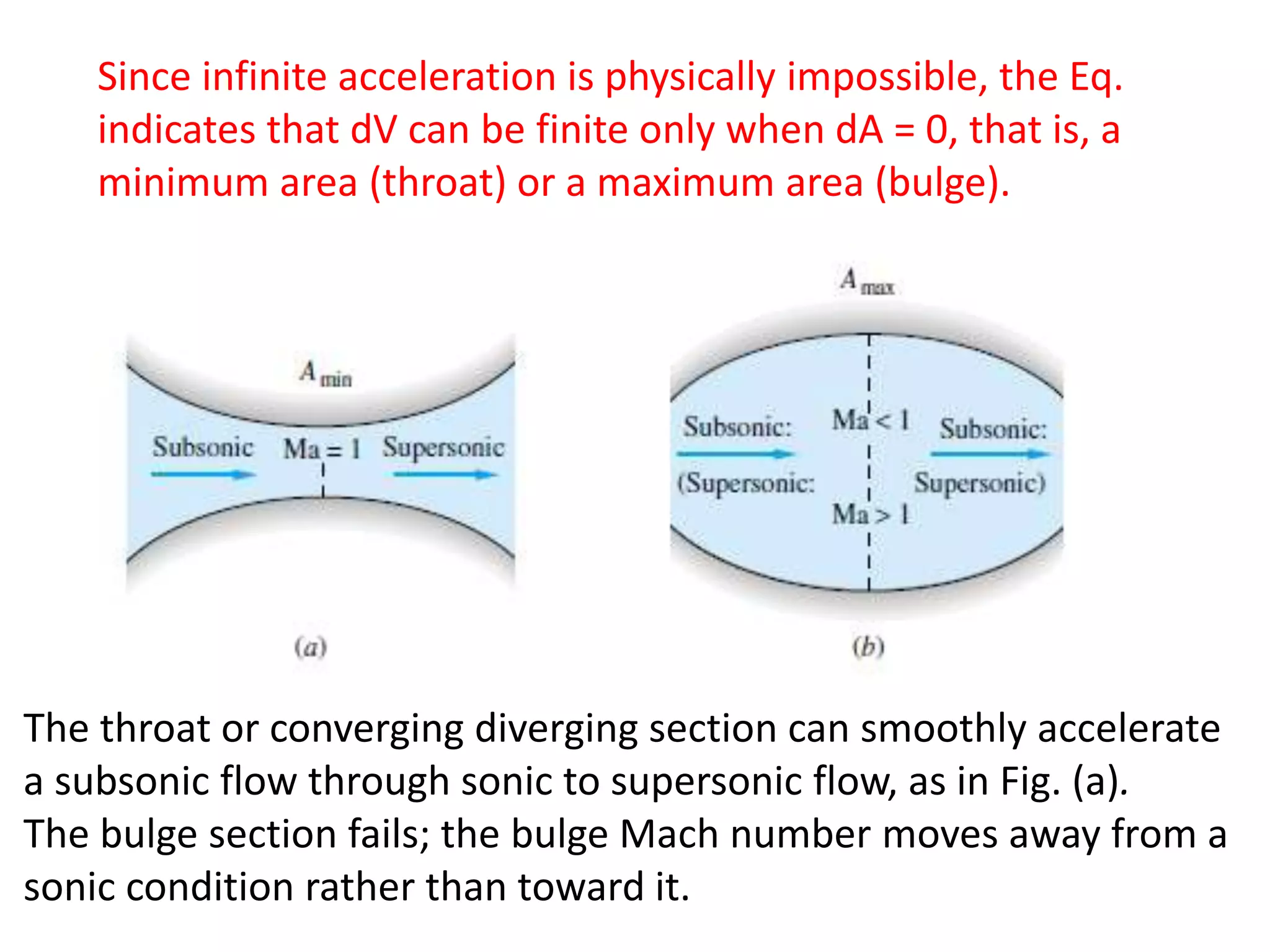
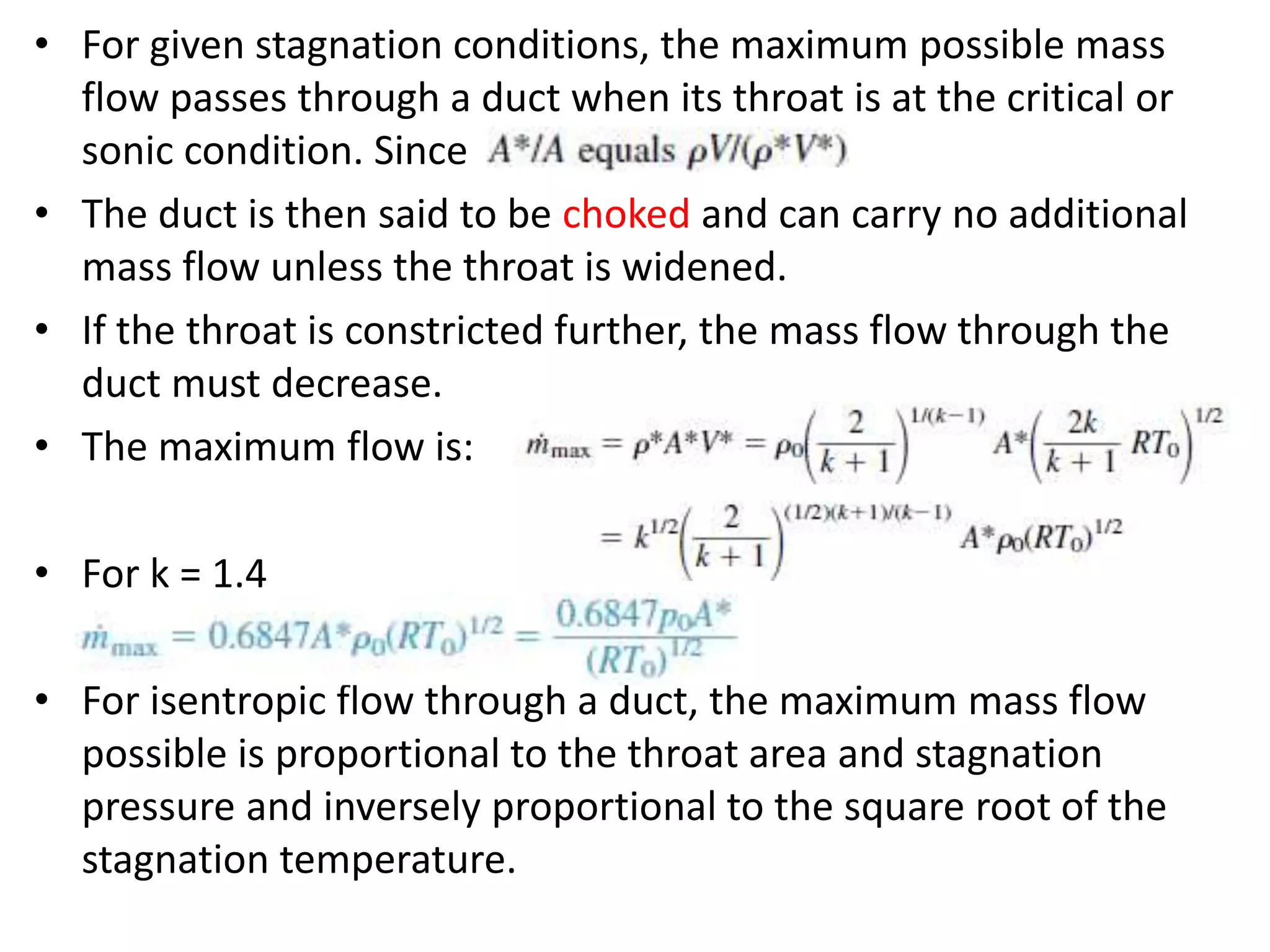
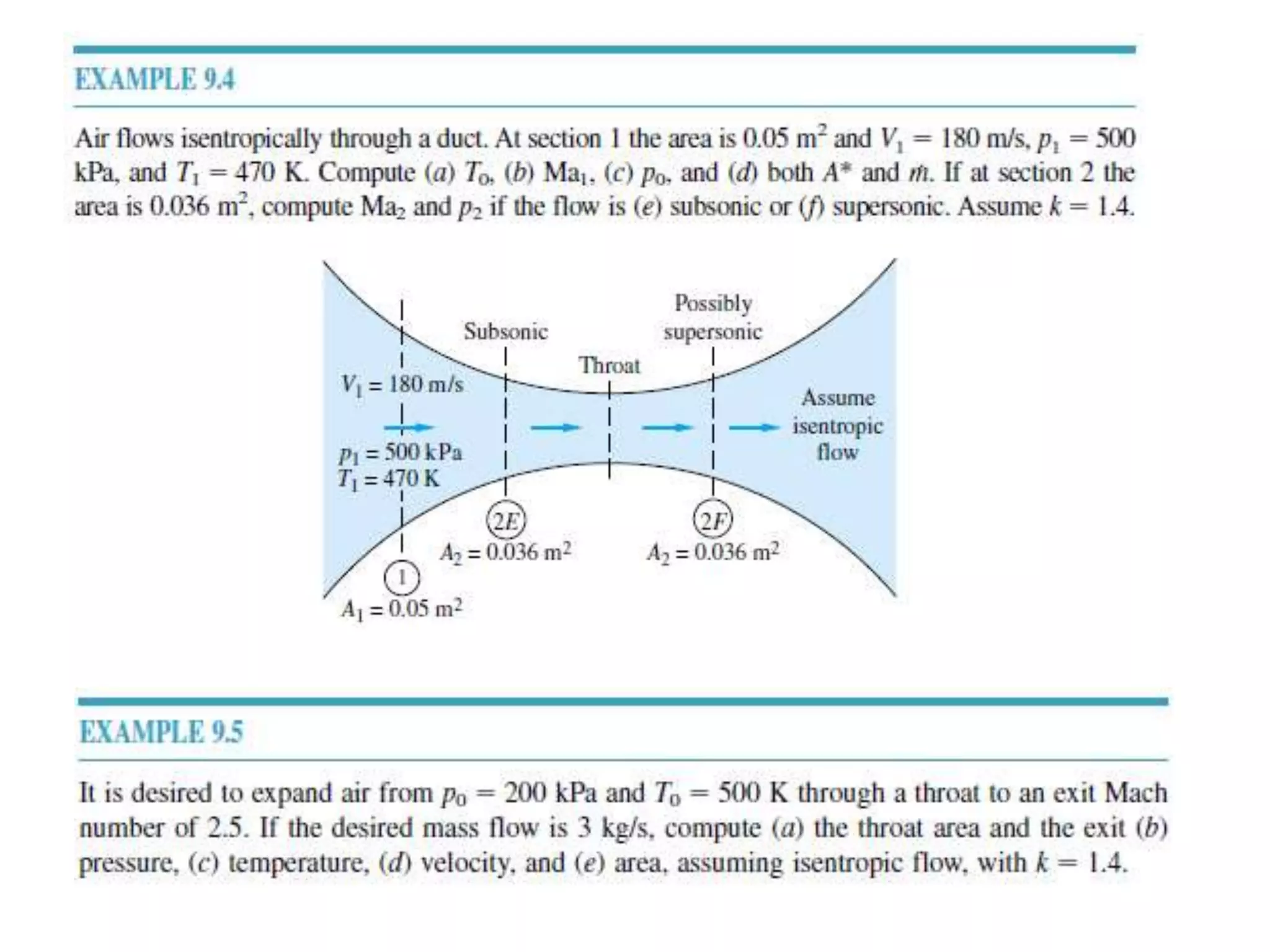
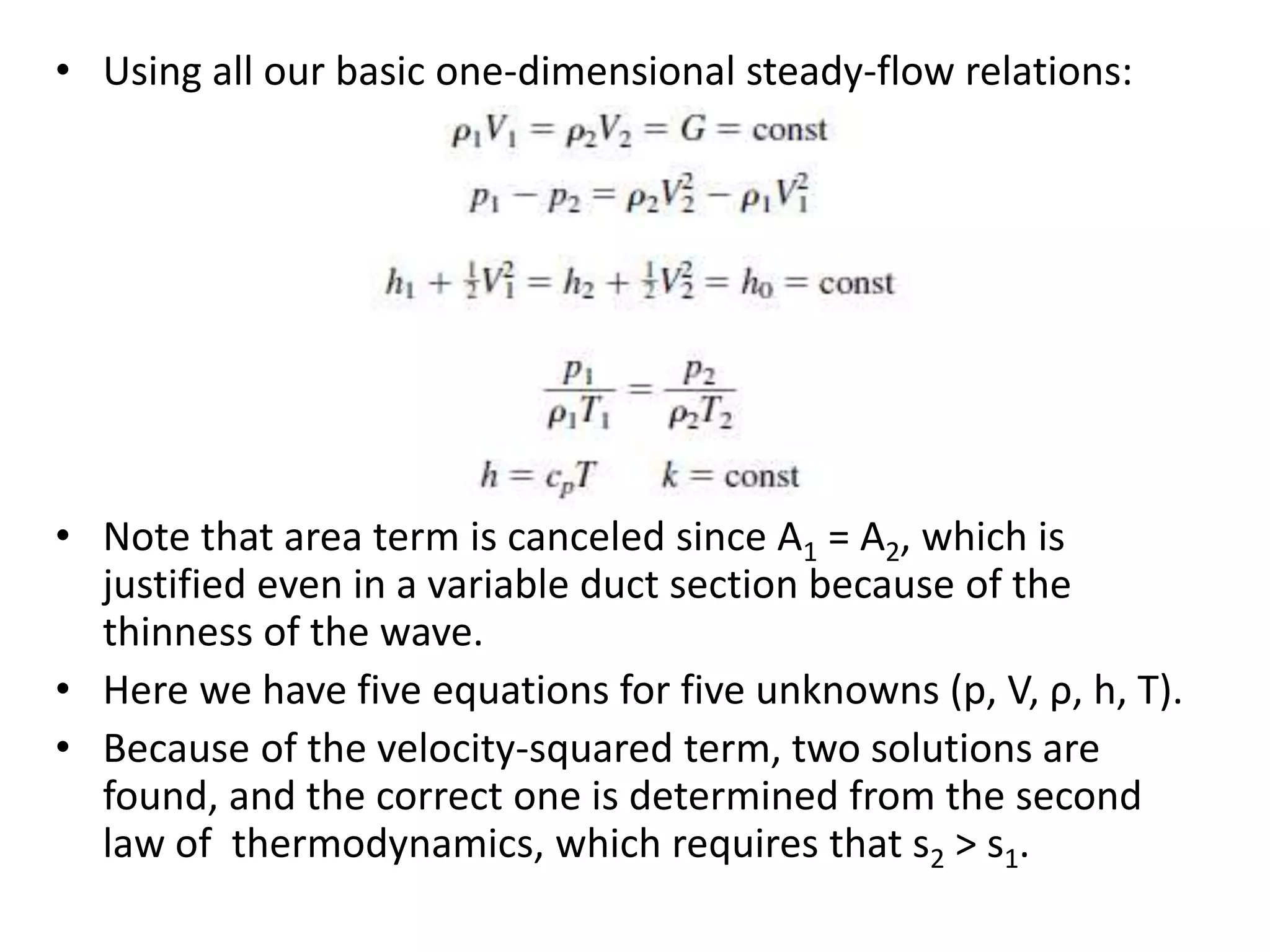
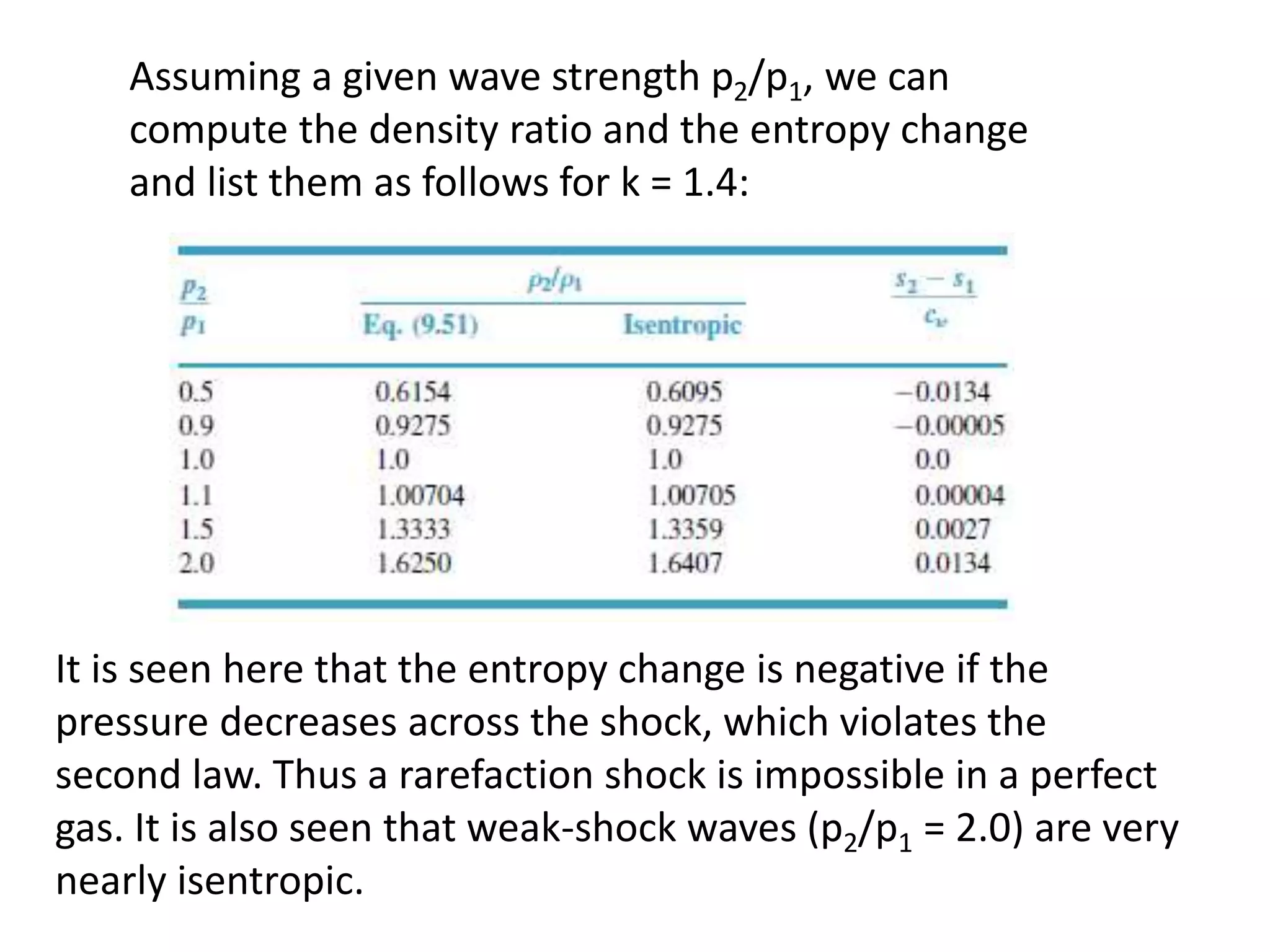
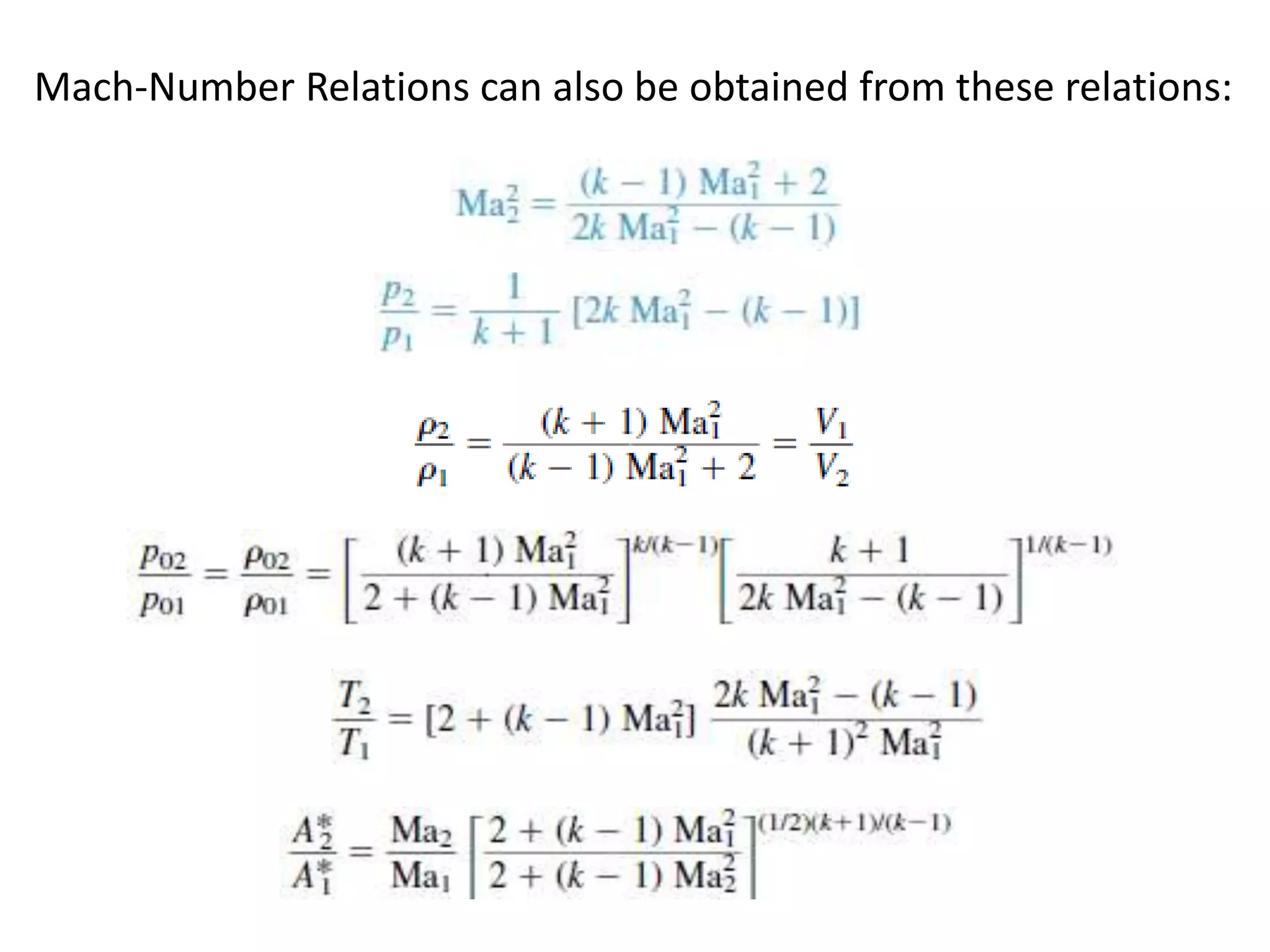
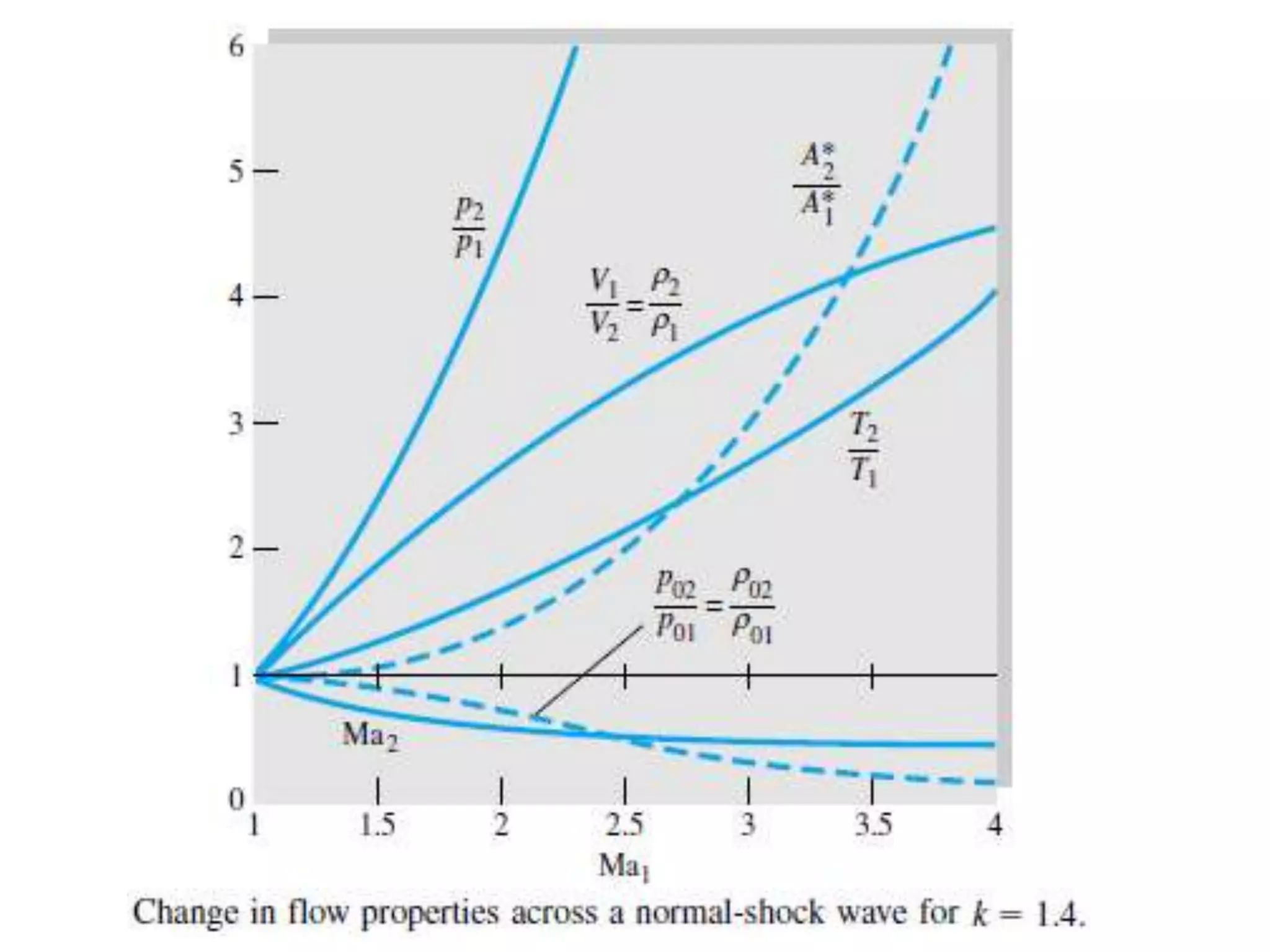
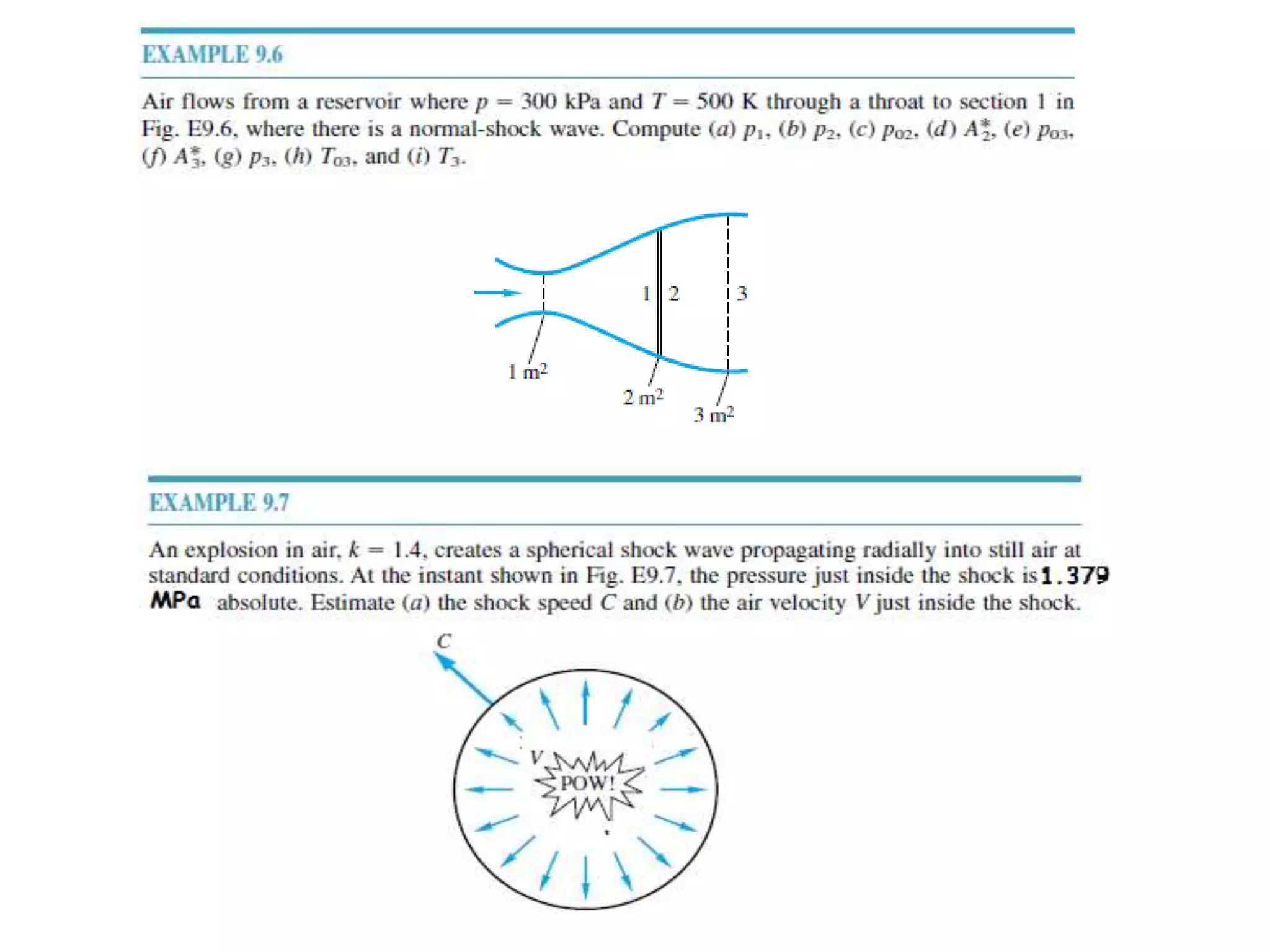
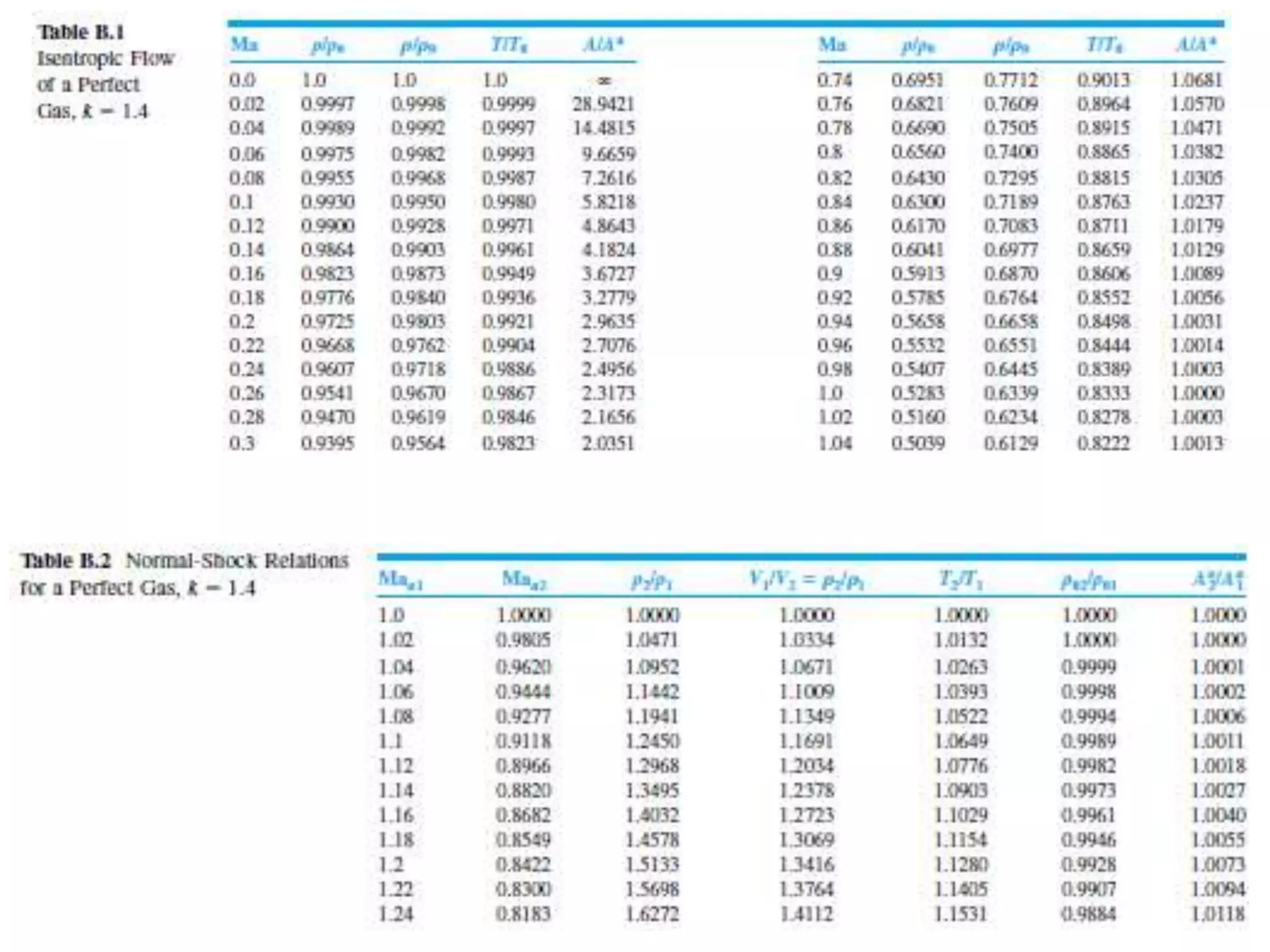
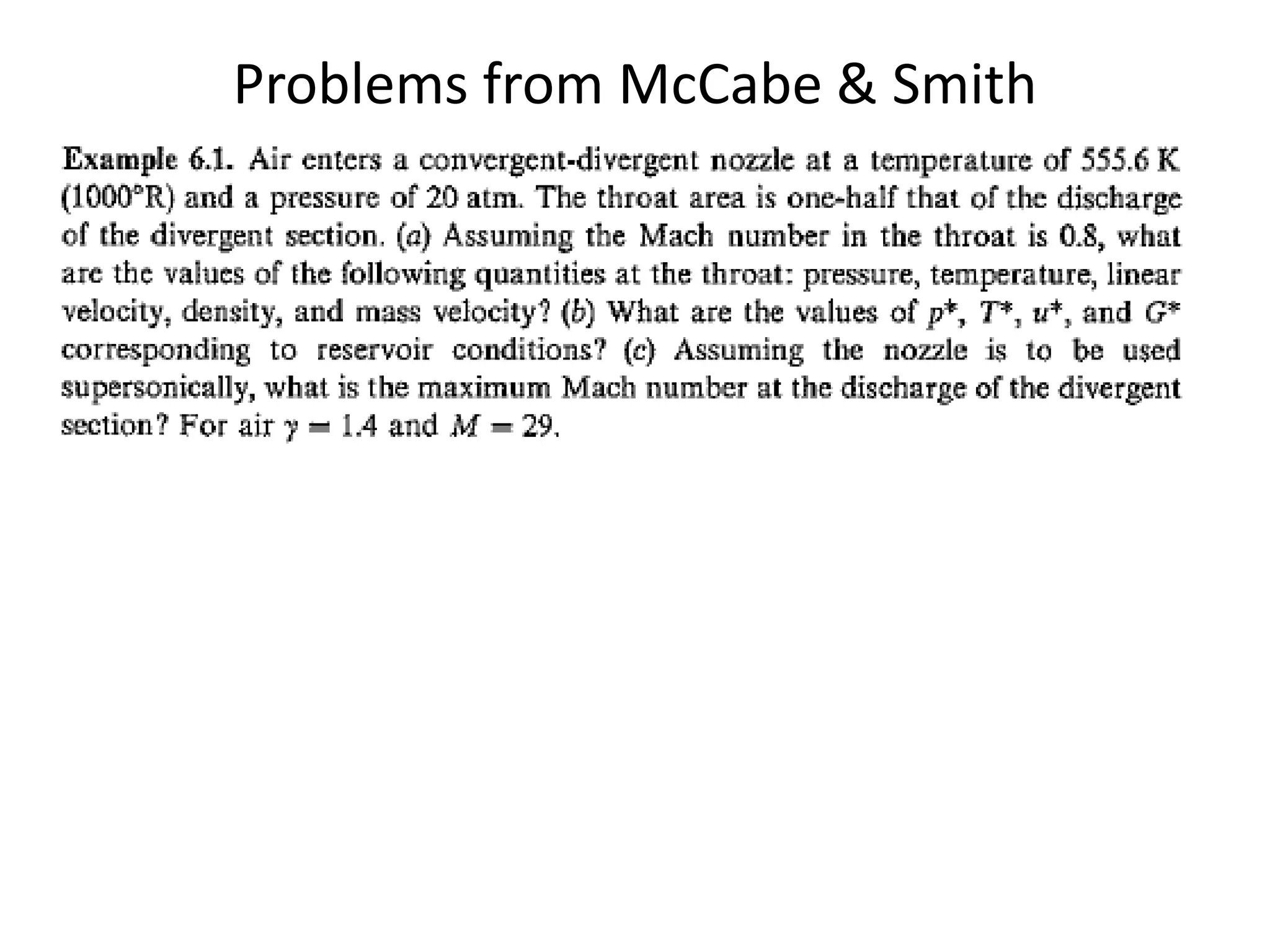
The document discusses compressible flows, highlighting their significance when fluid velocity approaches sound speed, leading to effects like choking and shock waves. It categorizes flow types based on Mach number and outlines the equations of state necessary to describe compressible gas behavior, including energy considerations for varying densities and temperatures. Additionally, it covers critical flow properties, the nature of shock waves, and the analytical methods for determining flow characteristics in compressible systems.