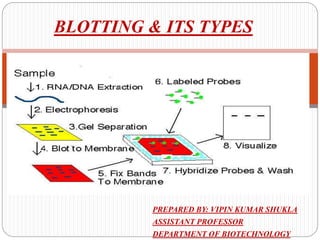
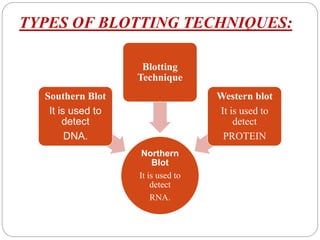
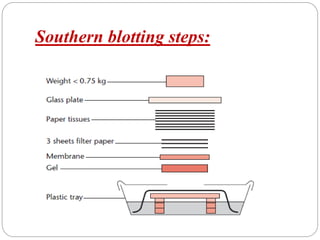
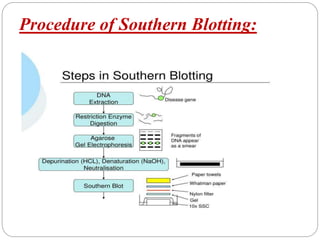
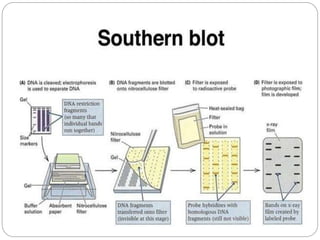
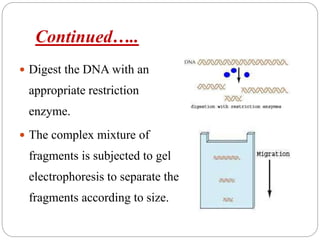
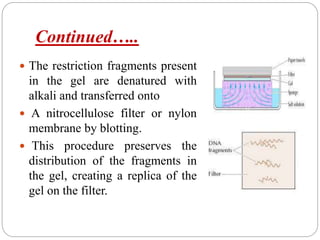
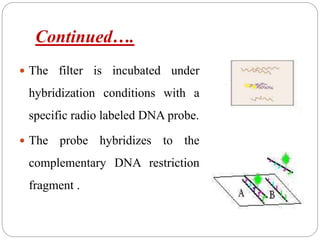
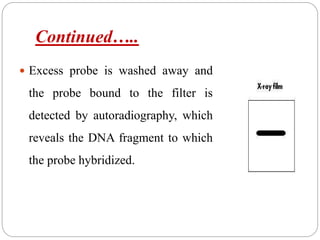
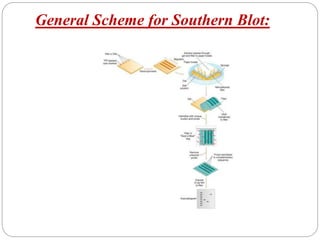
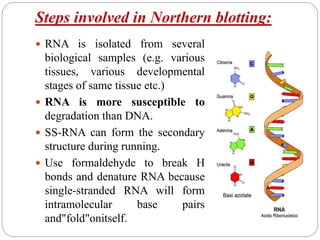
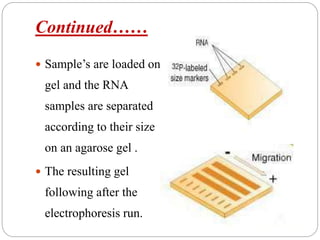
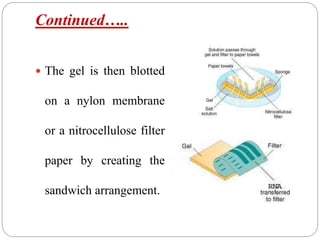
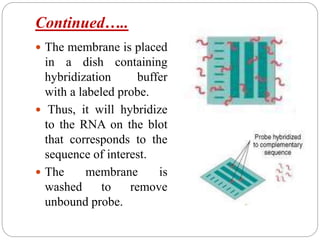
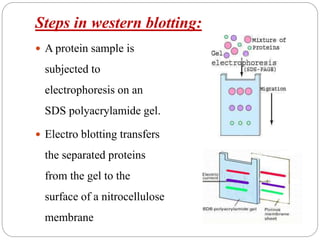
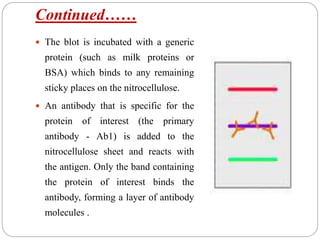
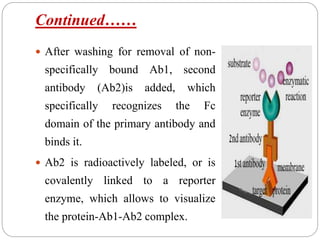
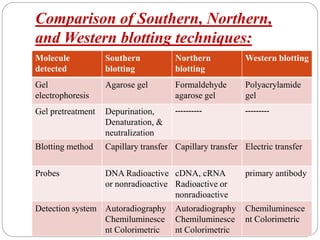
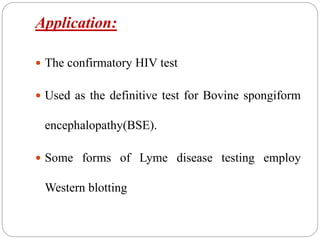
Blotting techniques such as Southern blotting, Northern blotting, and Western blotting allow for the detection of specific DNA, RNA, and protein sequences by transferring them from a separation gel onto a membrane and using probes to detect the targets. Southern blotting detects DNA using DNA probes, Northern blotting detects RNA using RNA or DNA probes, and Western blotting detects proteins using antibodies. These techniques are used for applications like detecting gene expression, RNA splicing, and confirming diseases.