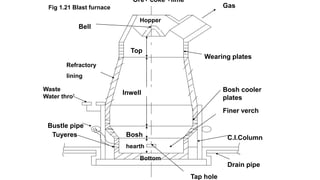
Manufacture of pig iron involves feeding iron ore, coke and limestone into the top of a blast furnace continuously. Air is blown into the bottom so that chemical reactions take place, melting the iron which is tapped from the bottom as molten pig iron. Pig iron is an impure form of iron containing carbon and other impurities. It is classified into grey pig iron which is high in silicon and forms graphite, and white pig iron which is low in silicon and forms cementite.