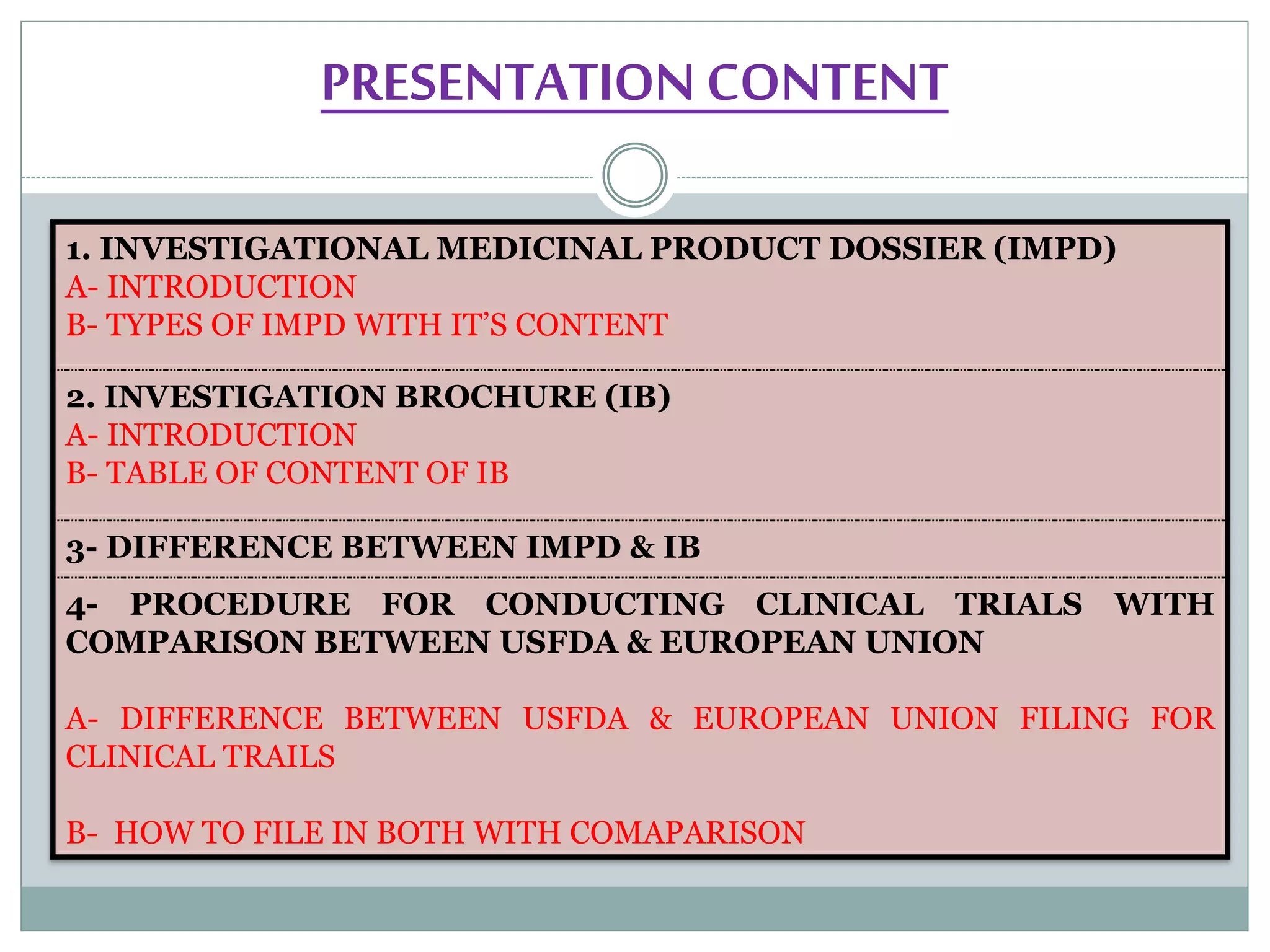
This document summarizes a presentation on Investigational Medicinal Product Dossier (IMPD) and Investigation Brochure (IB). The presentation covered:
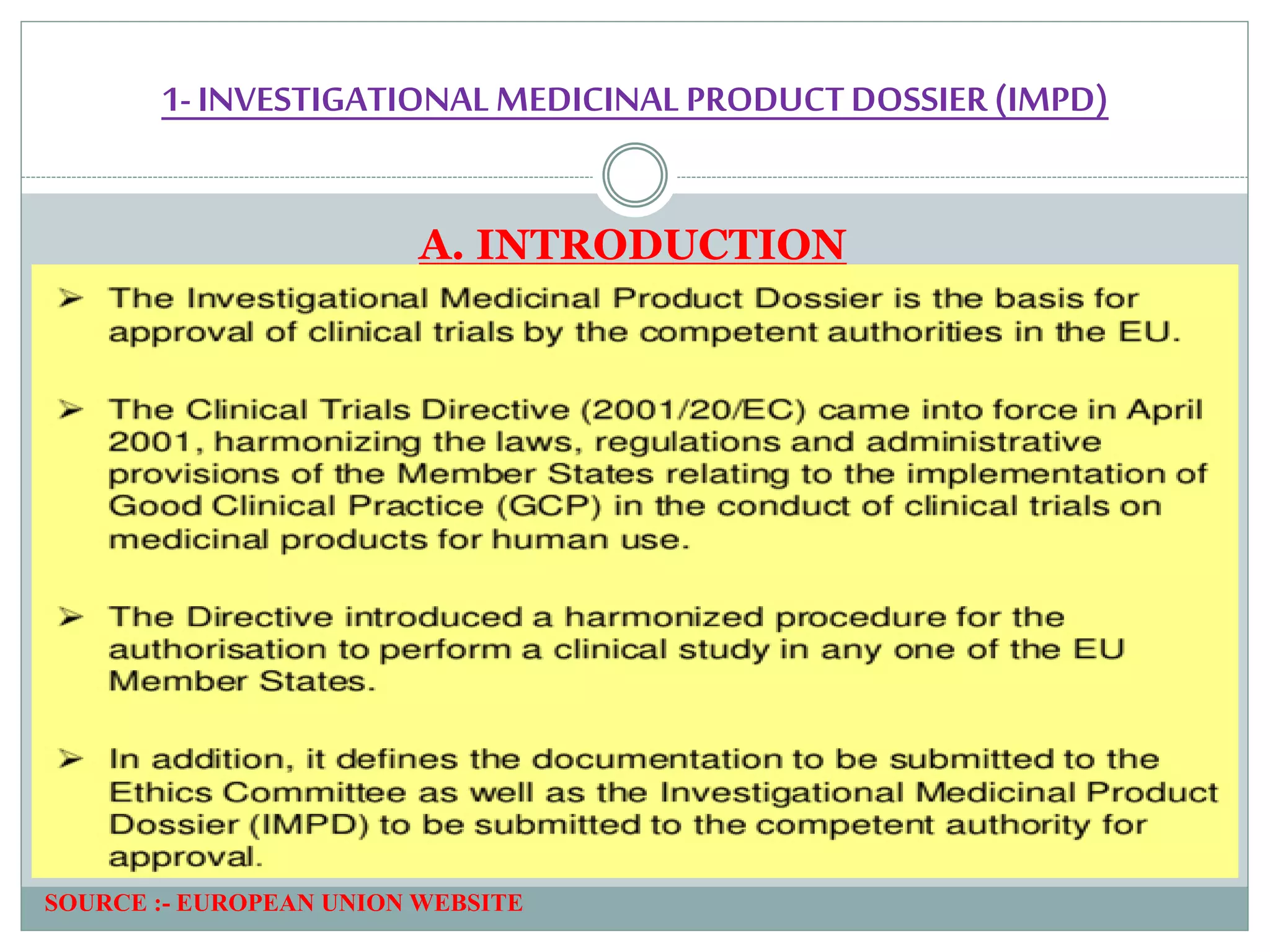
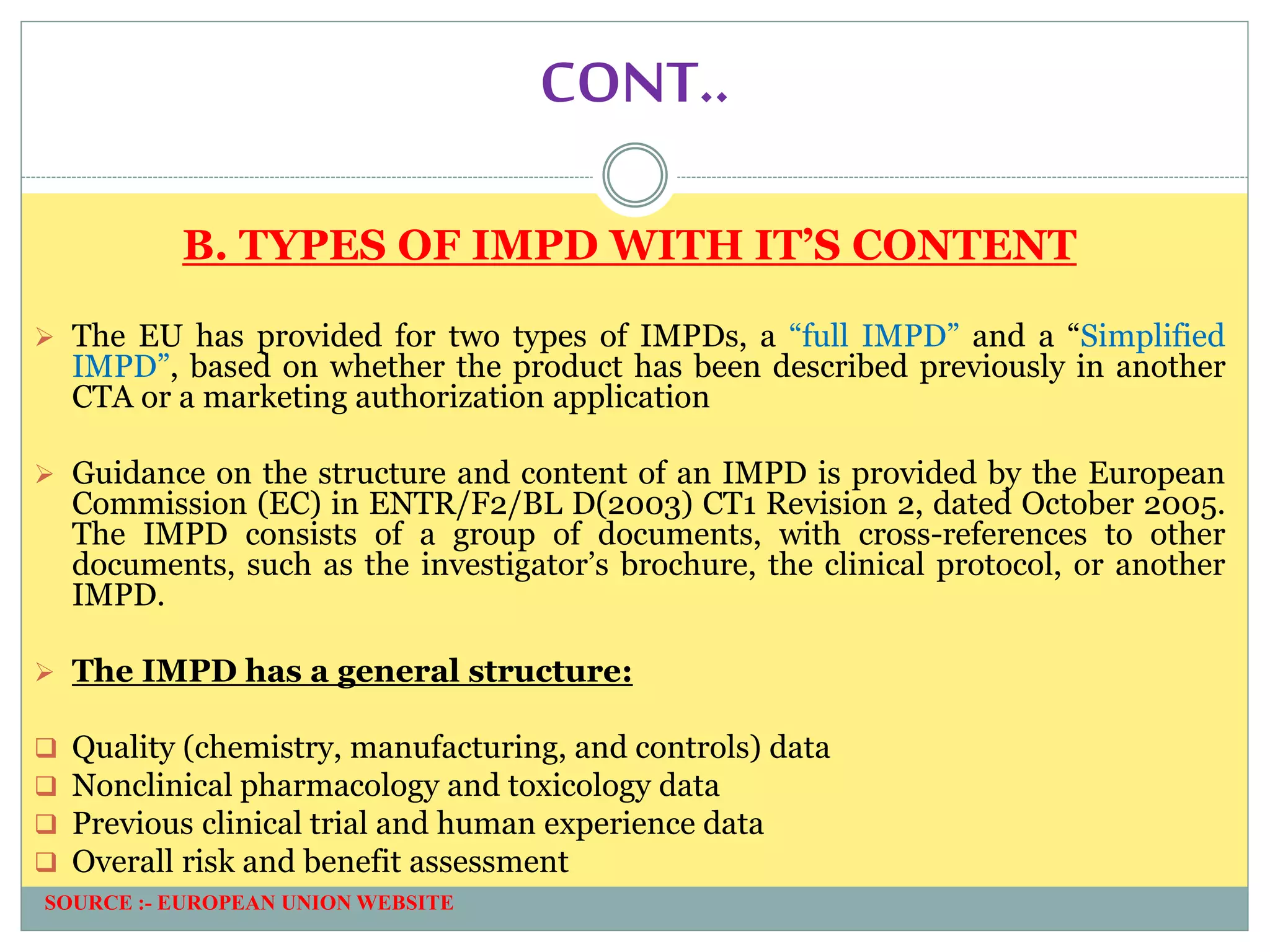
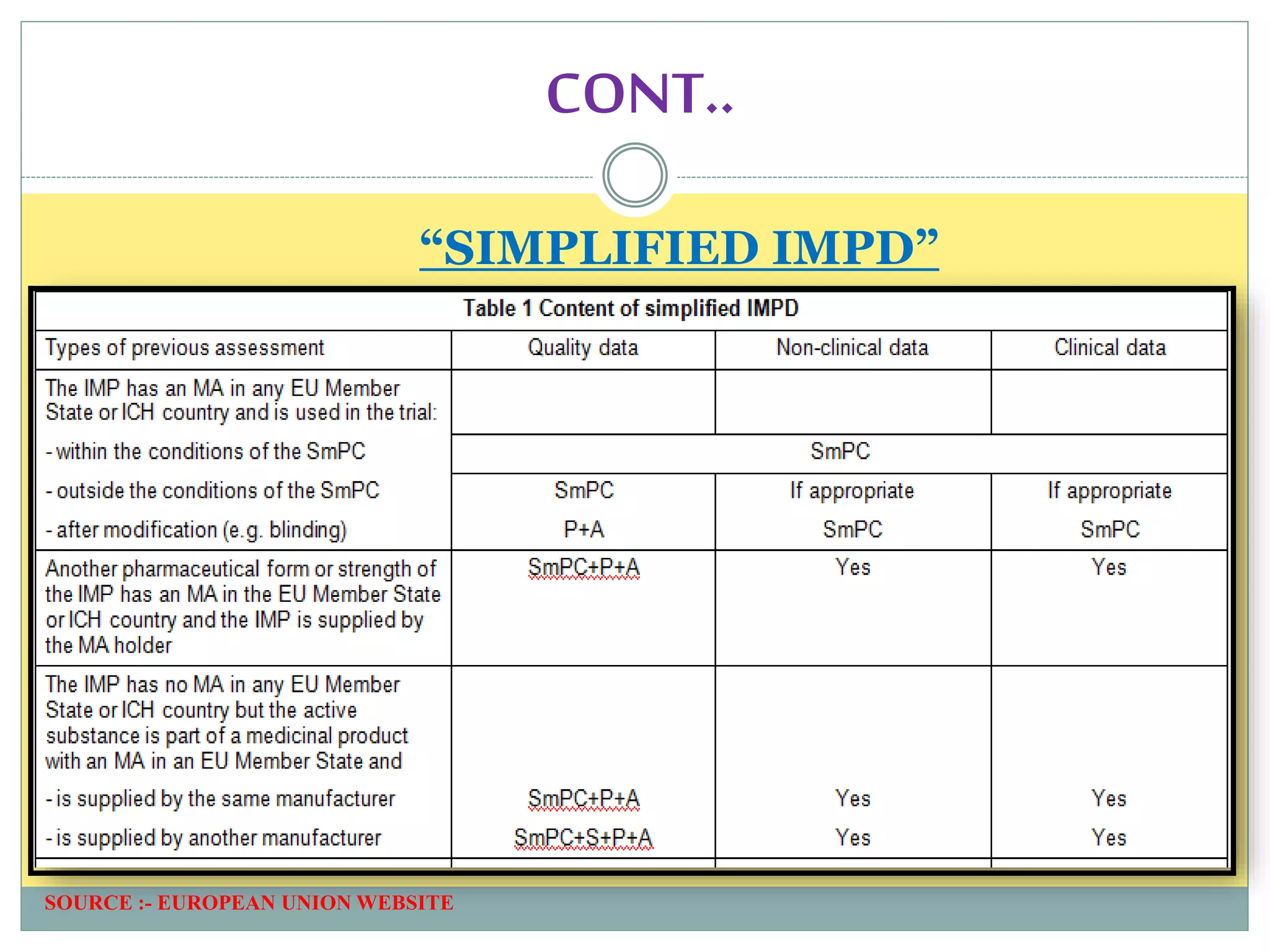
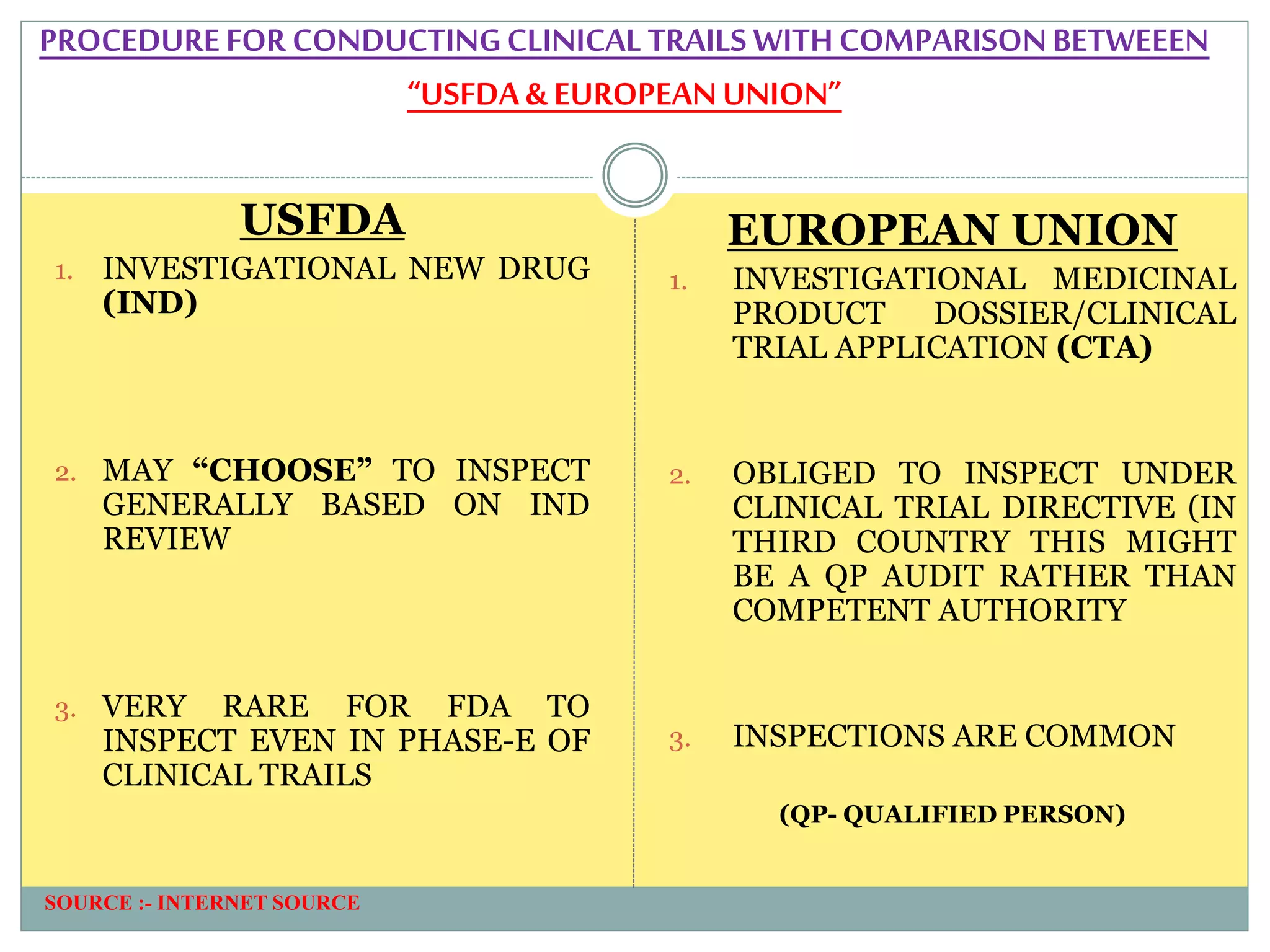
1. An introduction to IMPD, including that it is submitted to the European Union for clinical trial authorization and contains quality, nonclinical, and clinical data. Two types of IMPDs were described - full and simplified.
2. An introduction to the IB, which compiles relevant clinical and nonclinical data on investigational products to inform investigators. Key contents of an IB like title page, summary, and clinical data sections were outlined.
3. The differences between IMPD and IB, with IMPD focusing more on nonclinical development data and IB