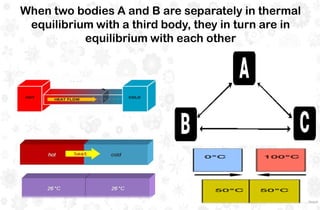
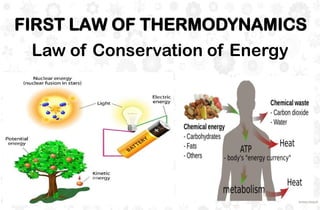
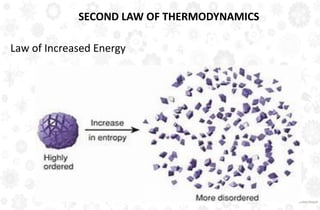
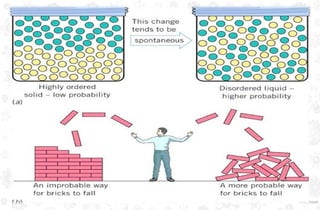
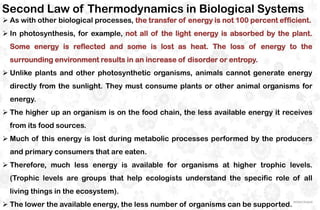
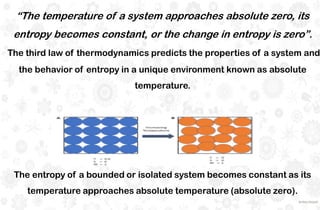
The document discusses the principles and laws of thermodynamics, including the zeroth, first, second, and third laws, along with their applications in biological systems. It emphasizes that energy cannot be created or destroyed, only transformed, and outlines how these energy transformations affect living organisms and ecosystems. The third law indicates that as systems approach absolute zero, entropy becomes constant, highlighting the need for constant energy input in living systems to maintain order.