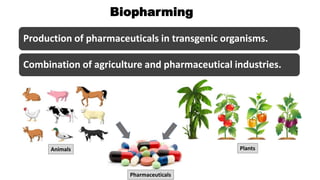
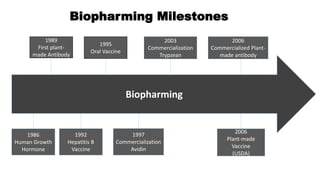
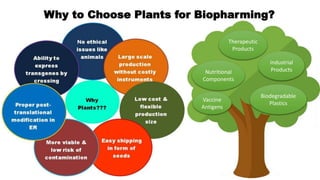
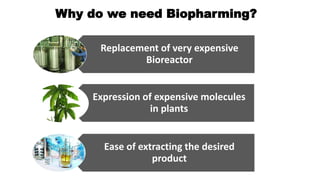
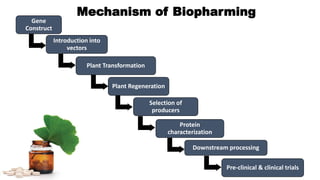
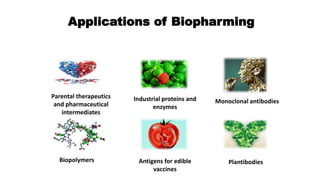
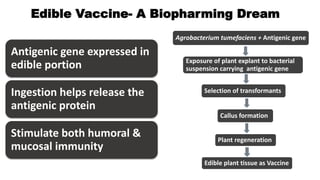
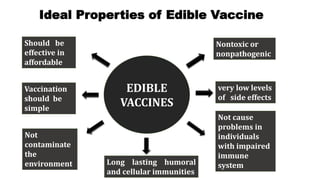
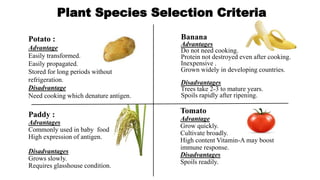
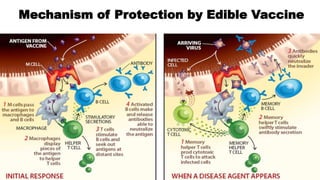
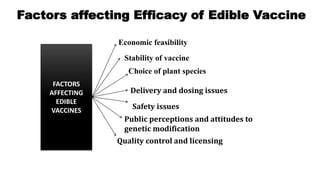
This document discusses biopharming and edible vaccines. It provides a brief history of biopharming milestones from 1989 to 2006. It explains that plants are a good choice for biopharming due to their ability to produce therapeutic products, nutritional components, vaccines, and industrial products in a cost effective manner. The document outlines the process of biopharming from gene construct development to clinical trials. It discusses some applications of biopharming including pharmaceuticals, industrial enzymes, monoclonal antibodies, and edible vaccines. Ideal properties and candidates for edible vaccines are presented, as well as the mechanisms and factors affecting their protection and efficacy. Advantages and disadvantages of edible vaccines are compared. The document concludes