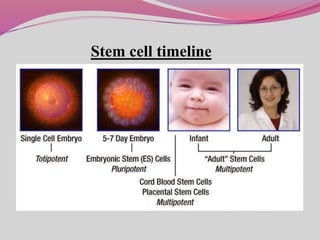
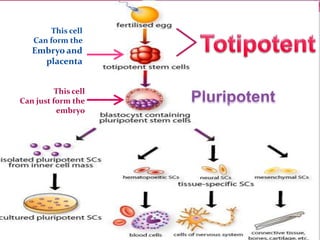
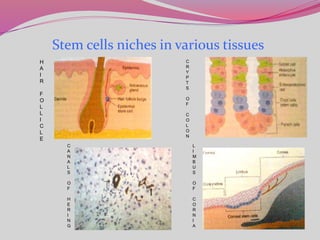
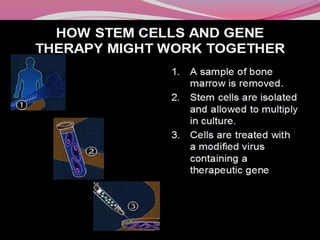
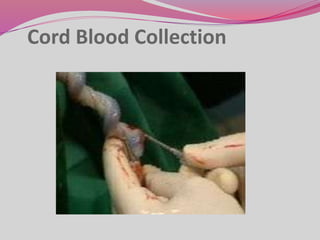
The document discusses various types of stem cells, including embryonic and adult stem cells, their unique properties, and potential applications in regenerative medicine. It details the process of extracting and culturing stem cells, as well as their roles in treating diseases and conditions such as cancer and neurological disorders. Additionally, it outlines the differences between stem cell sources and highlights the importance of umbilical cord blood as a regenerative resource.