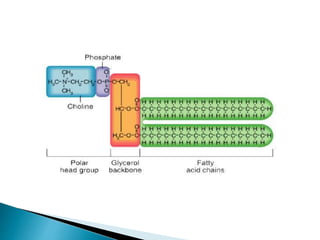
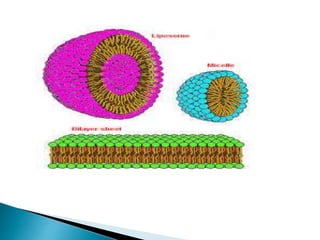
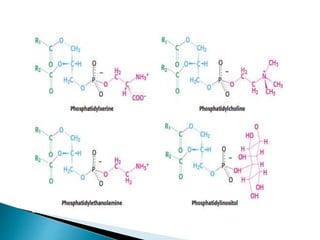
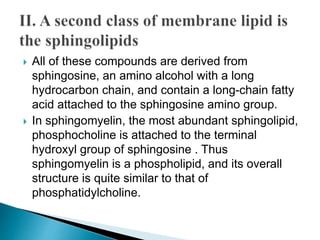
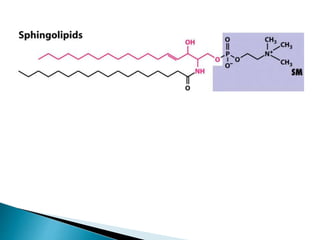
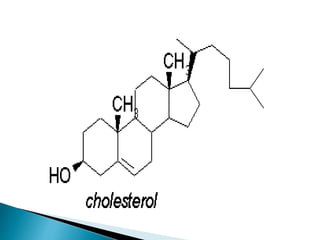
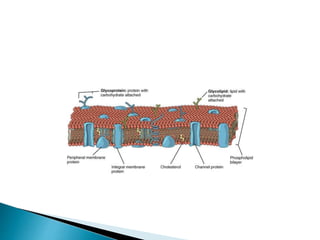
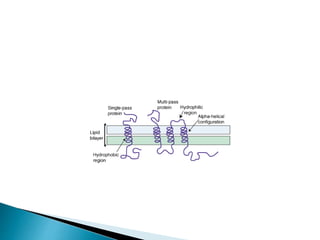
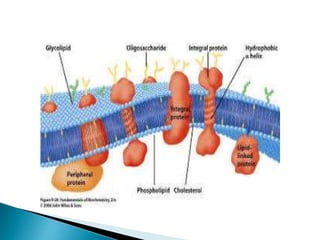
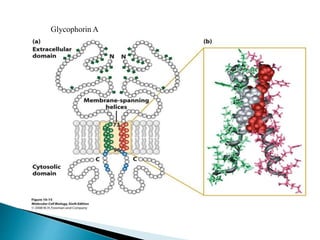
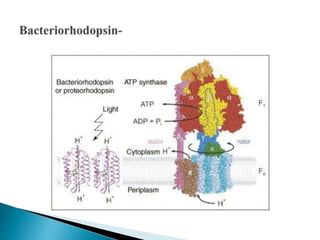
This document discusses the structure and composition of bio-membranes. It states that bio-membranes are composed primarily of phospholipids that spontaneously form a bilayer structure. The phospholipids are amphipathic, with a hydrophobic tail and hydrophilic head. This allows the tails to interact at the membrane interior, separating the hydrophilic exterior into cytosolic and extracytosolic leaflets. Membranes also contain proteins and sterols that modulate membrane properties and functions. Integral membrane proteins span the bilayer, while peripheral proteins are attached to surfaces.