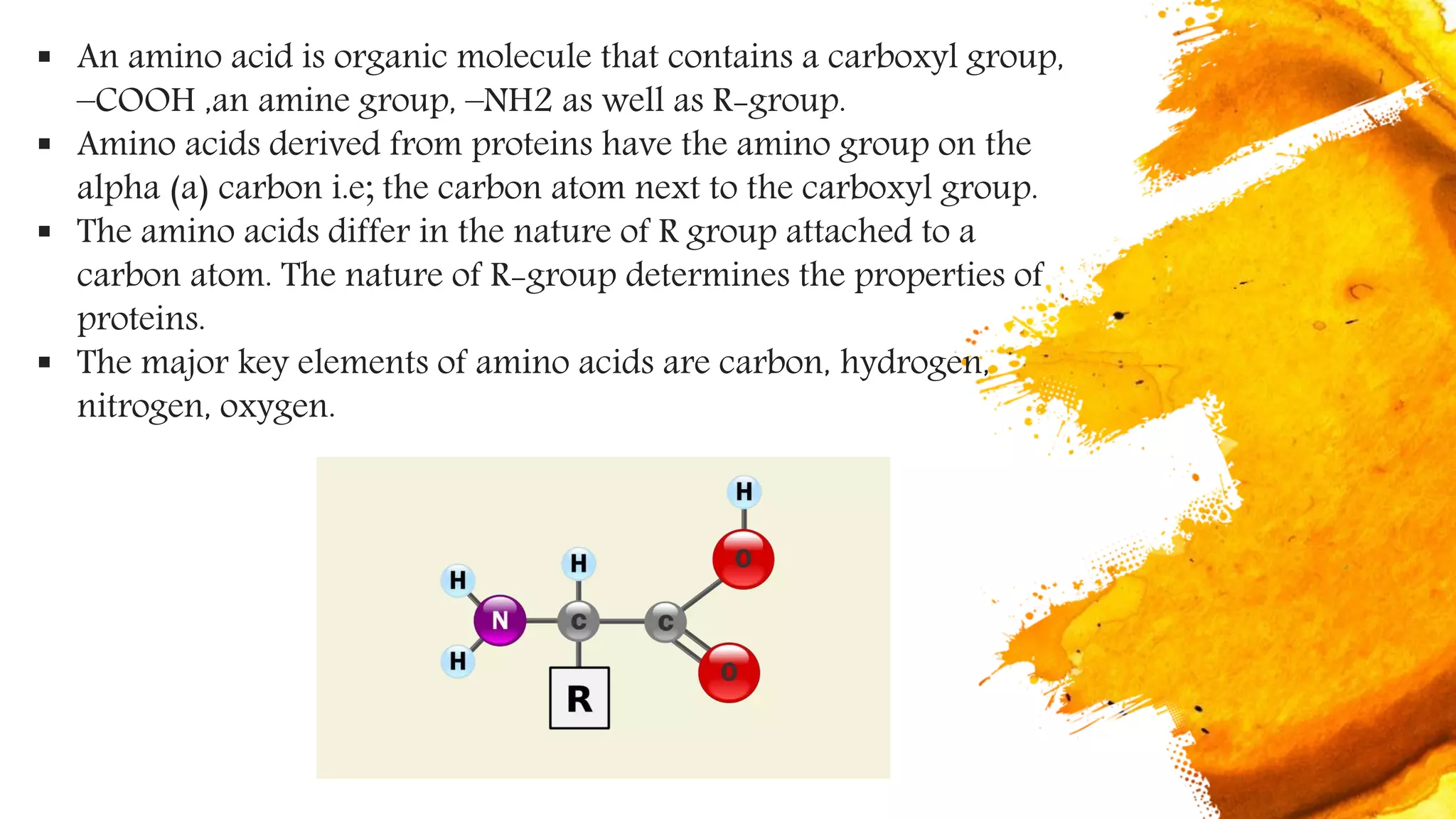
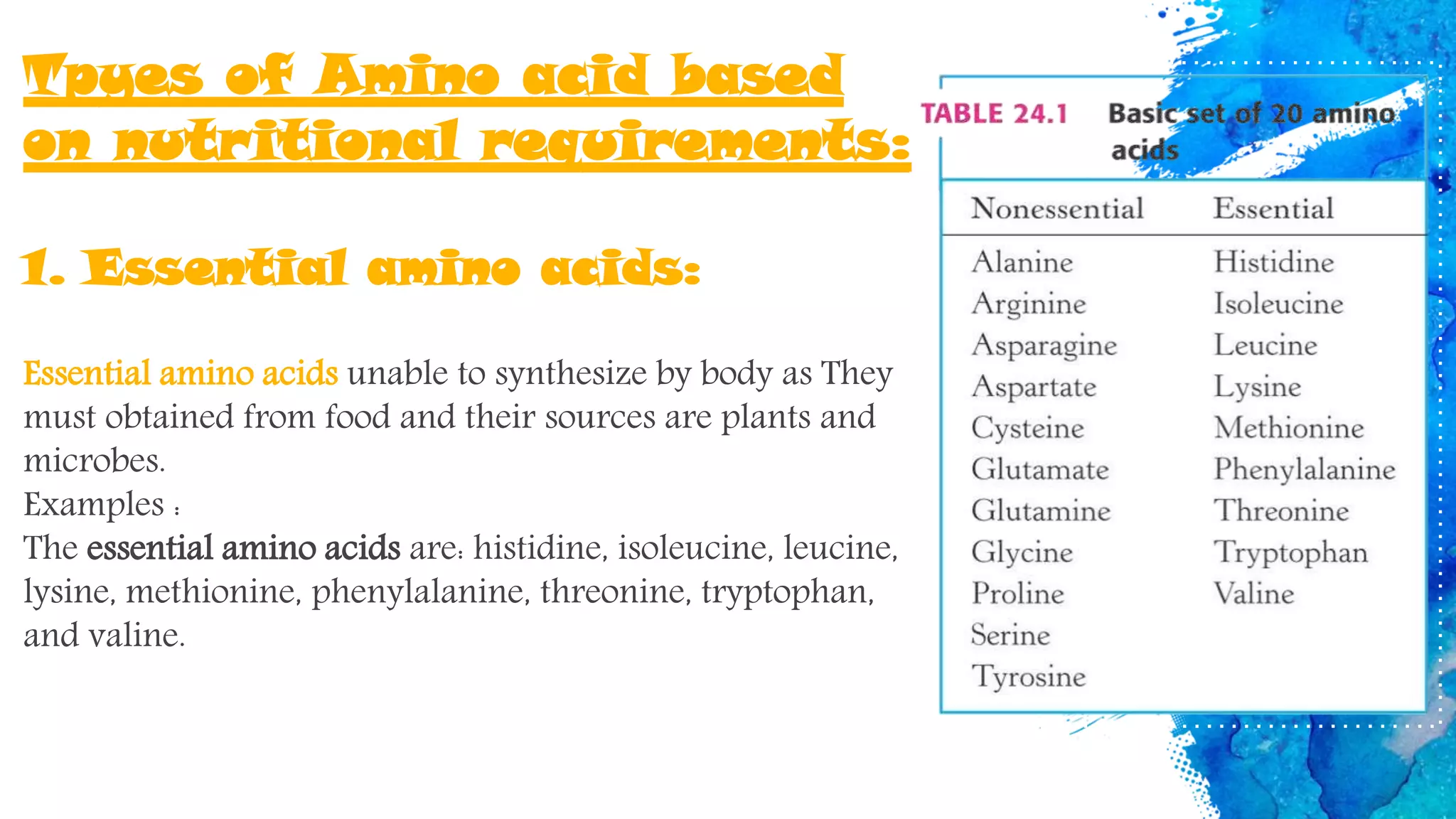
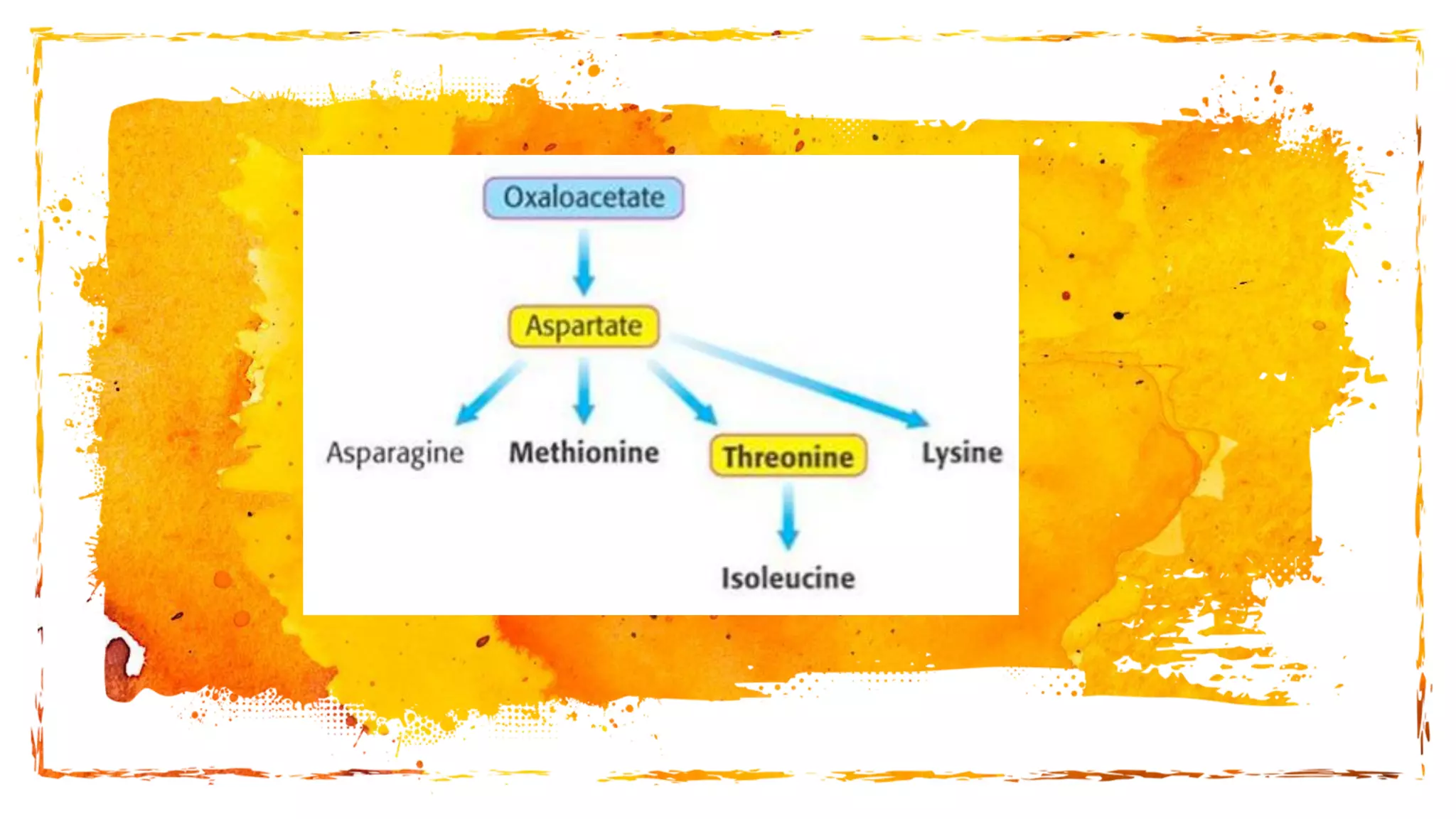
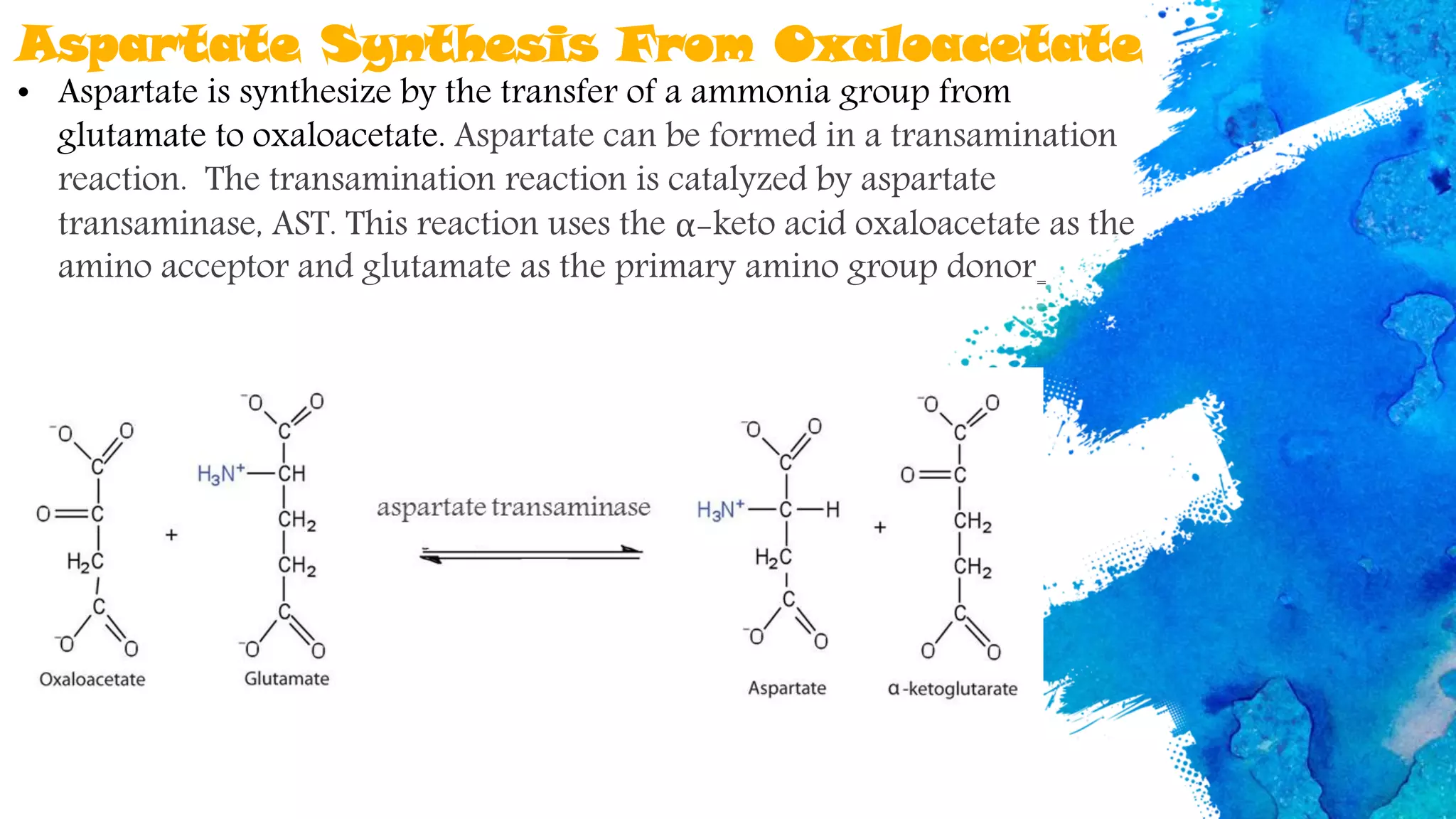
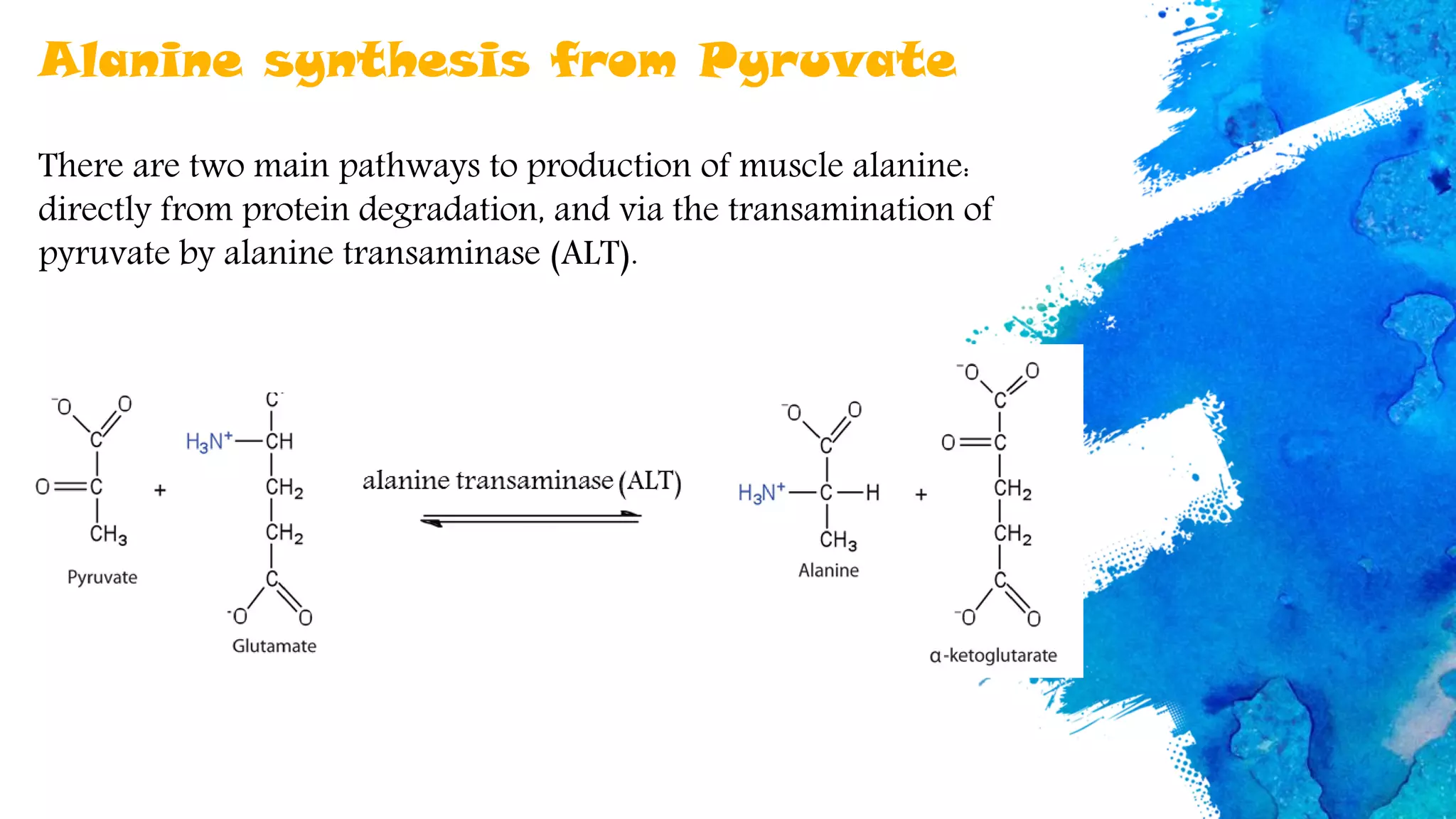
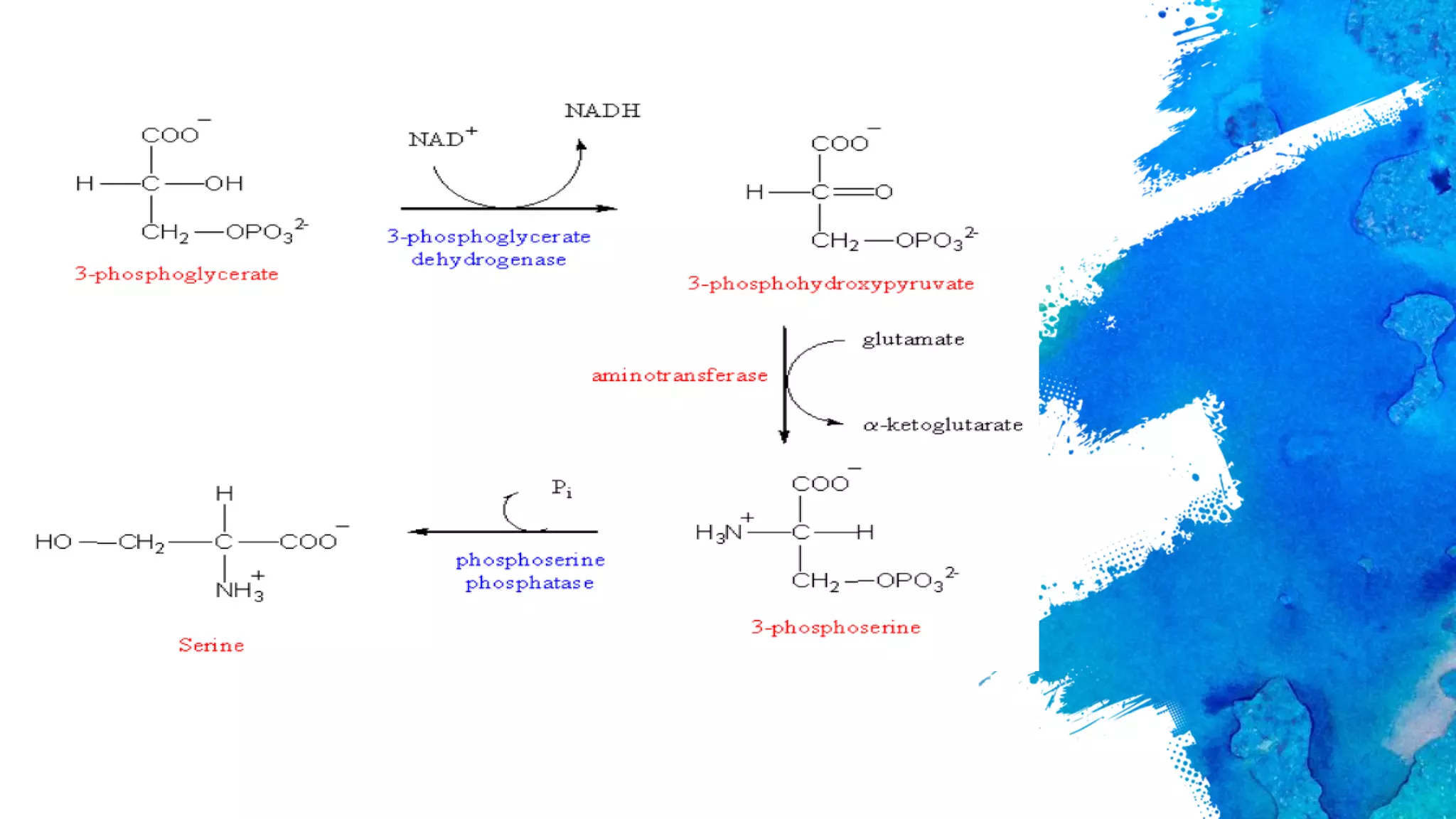
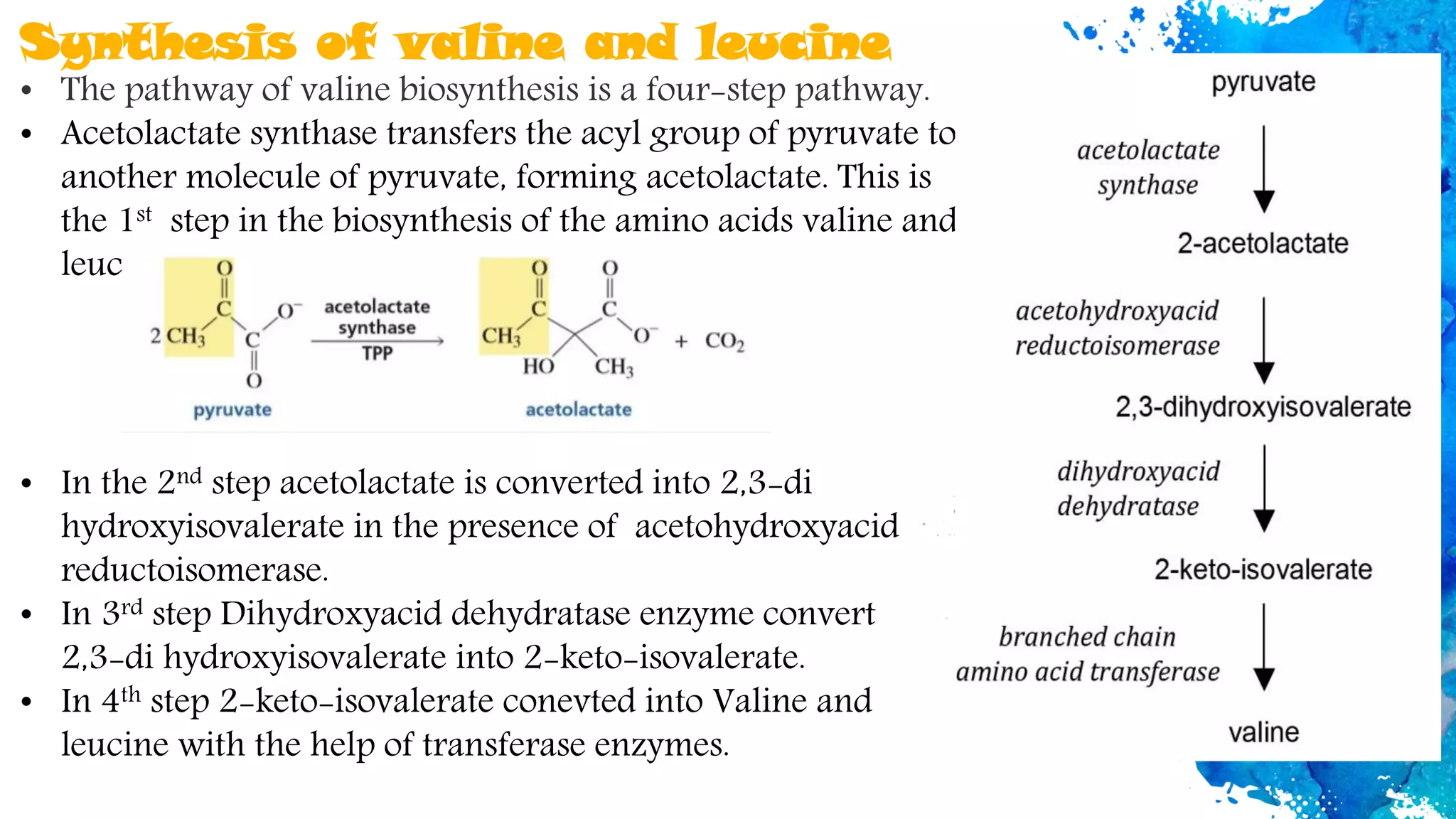
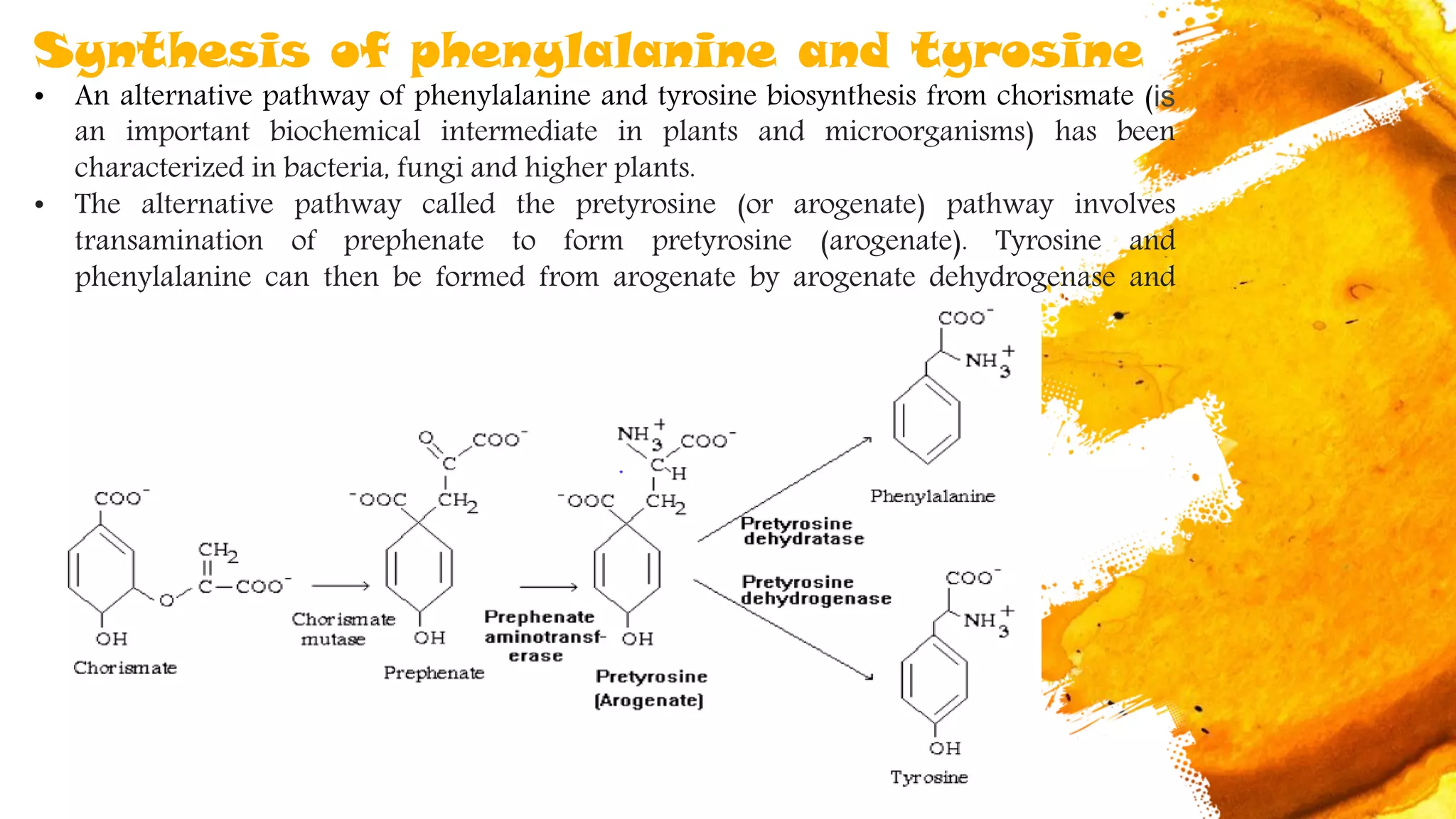
Amino acids are organic compounds that are the building blocks of proteins. There are two types - essential amino acids that must be obtained through diet as the body cannot synthesize them, and non-essential amino acids that the body can synthesize from other compounds if not obtained in diet. The document discusses the biosynthesis pathways of both essential and non-essential amino acids from common metabolic precursors like pyruvate, oxaloacetate, and glutamate. Key enzymes involved in amino acid synthesis include glutamate dehydrogenase, glutamine synthetase, aspartate transaminase, and others.

















![Dehydratases are a group of lyase enzymes
that form double and triple bonds in a
substrate through the removal of water
Dehydrogenases are a group of biological
catalysts (enzymes) that mediate in
biochemical reactions removing hydrogen
atoms [H] instead of oxygen [O]](https://image.slidesharecdn.com/biosynthesisofaminoacid-200825092005/75/Biosynthesis-of-amino-acid-essential-and-non-essential-26-2048.jpg)