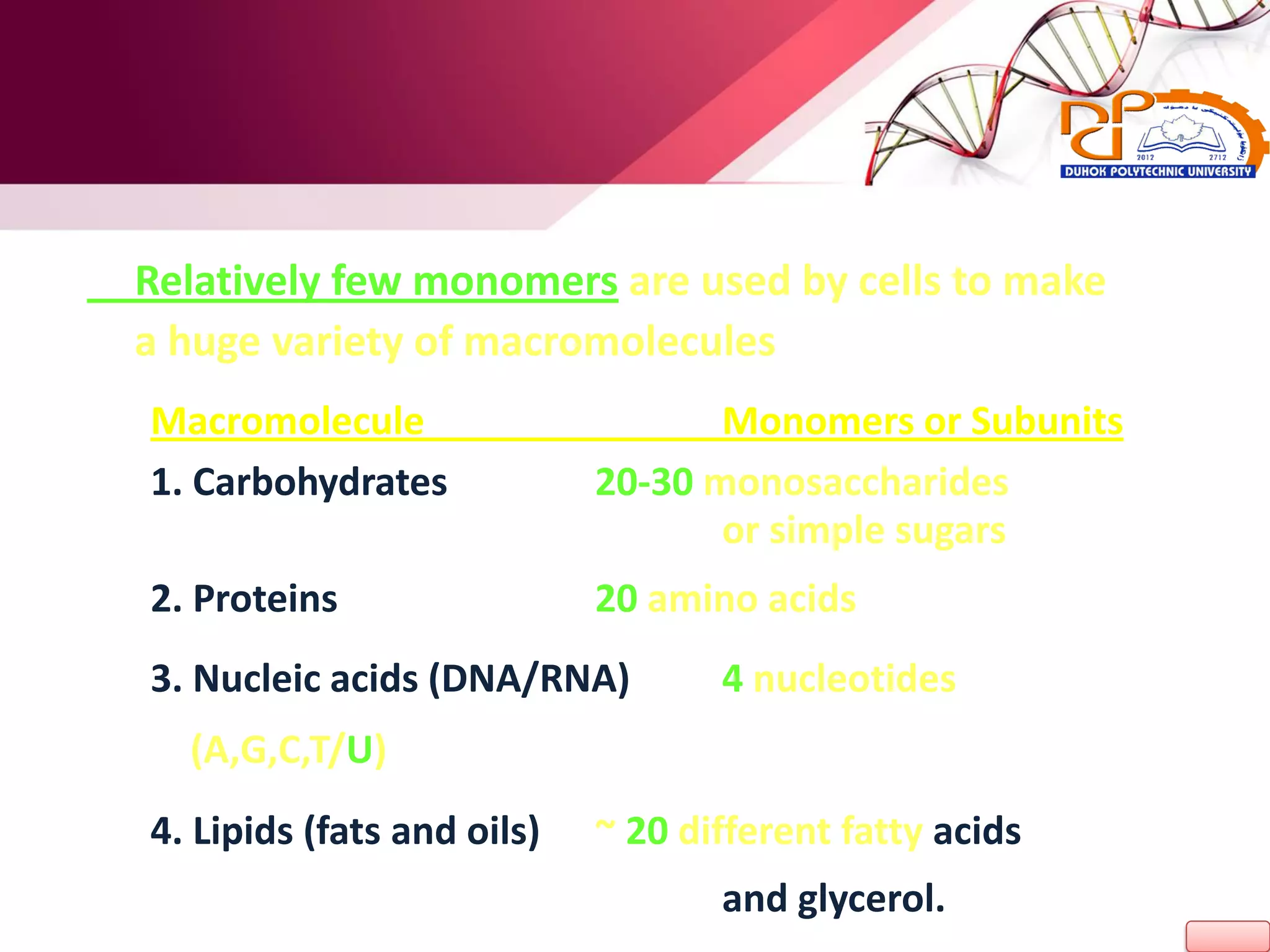
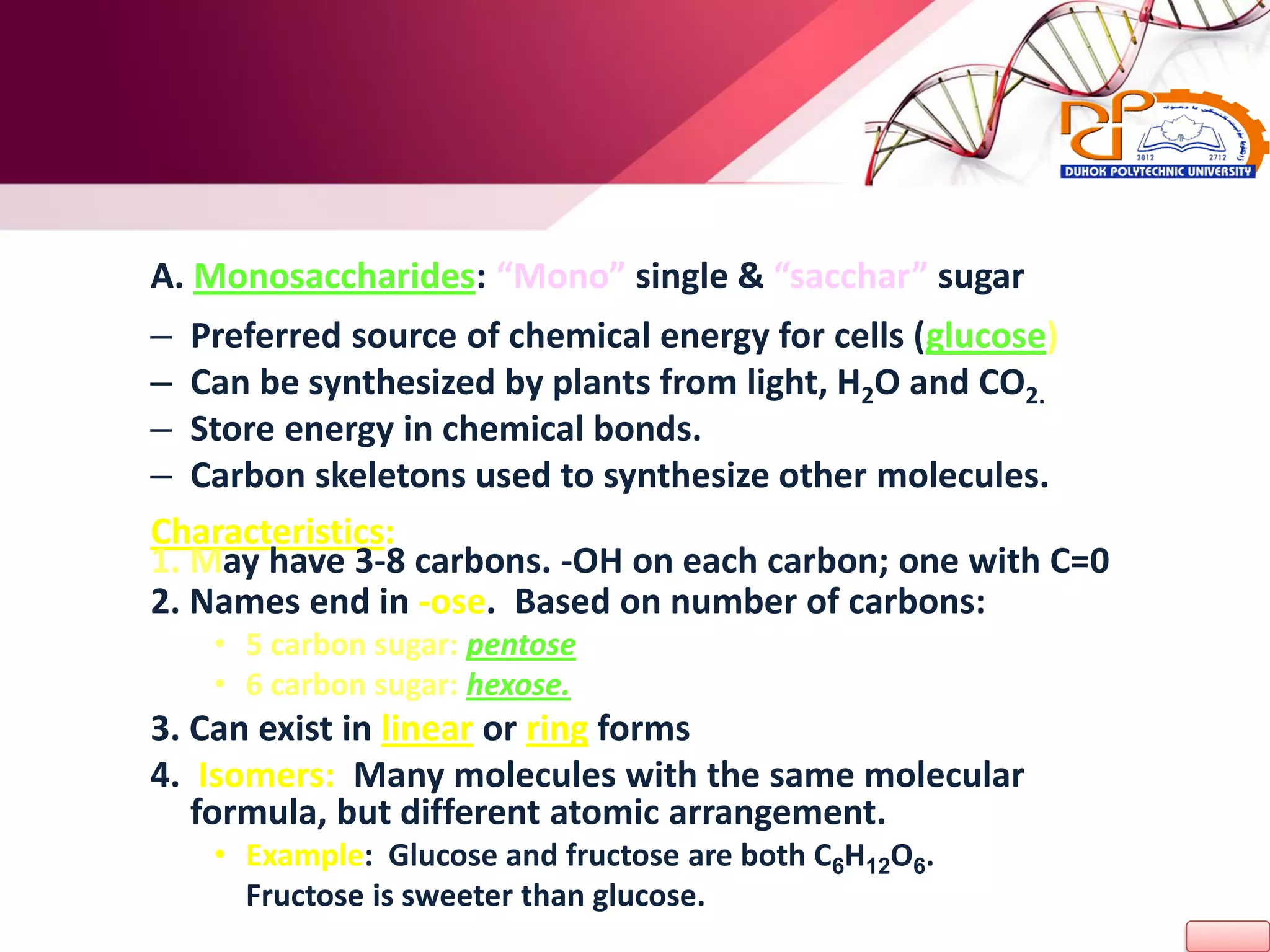
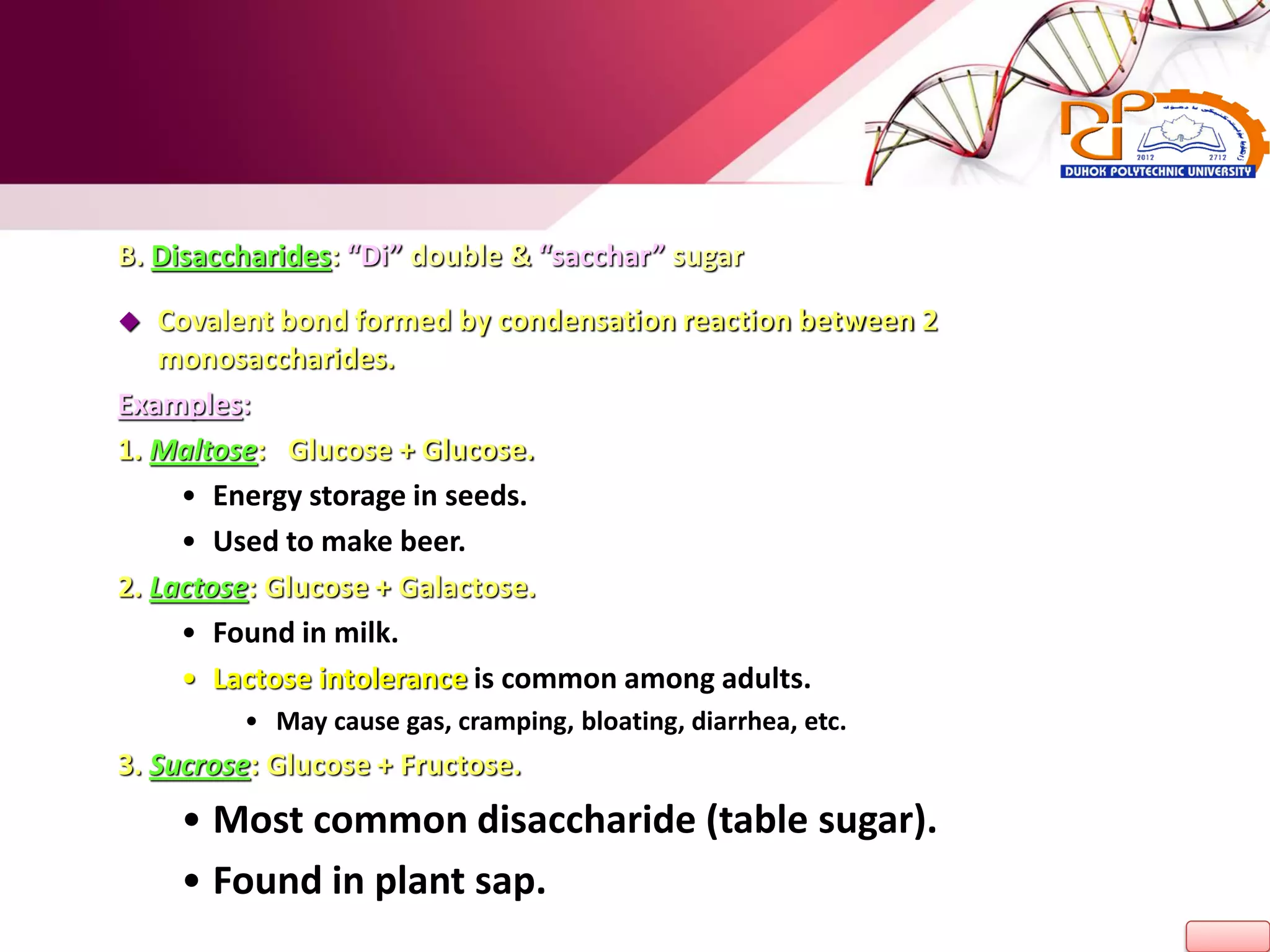
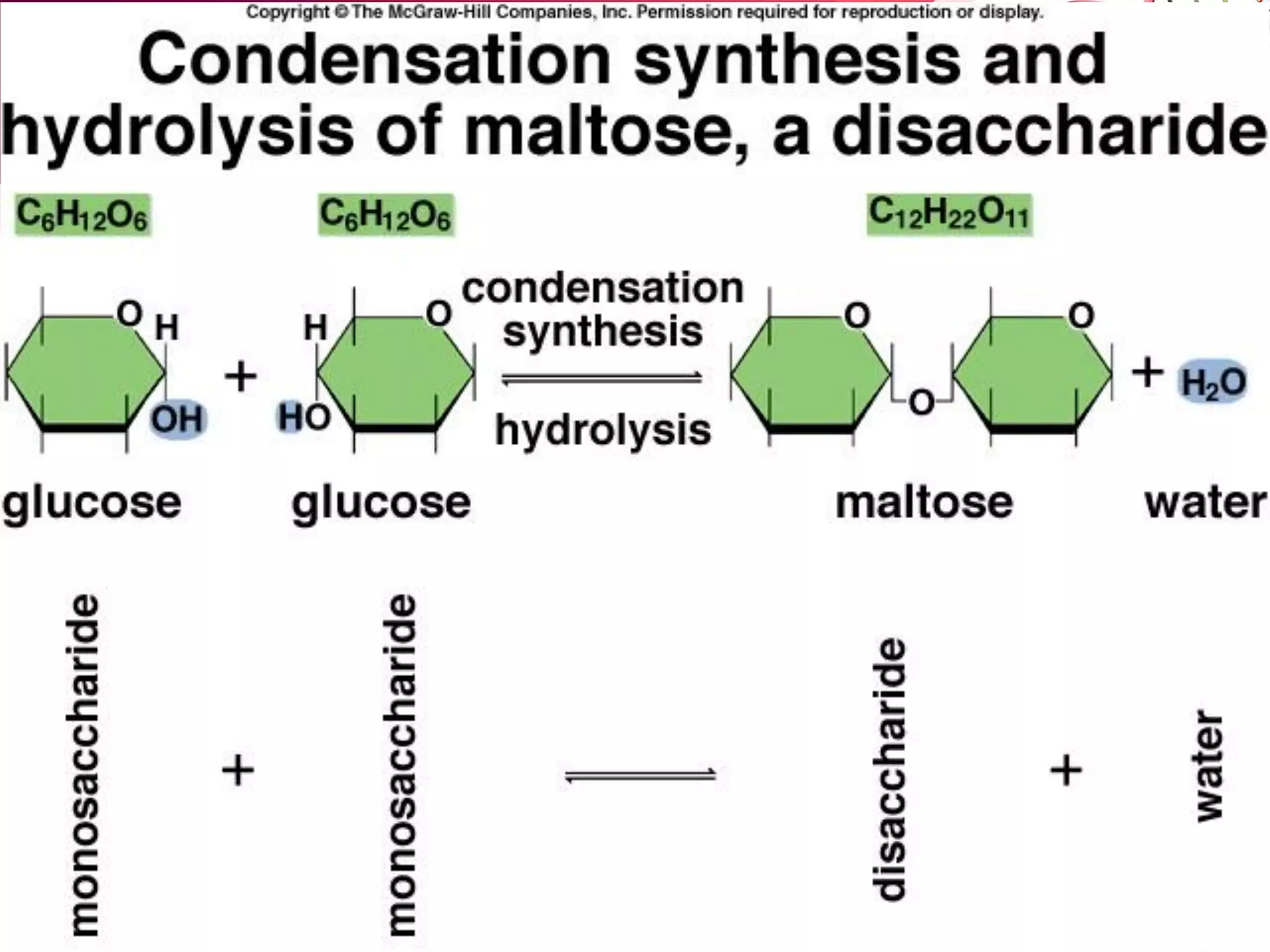
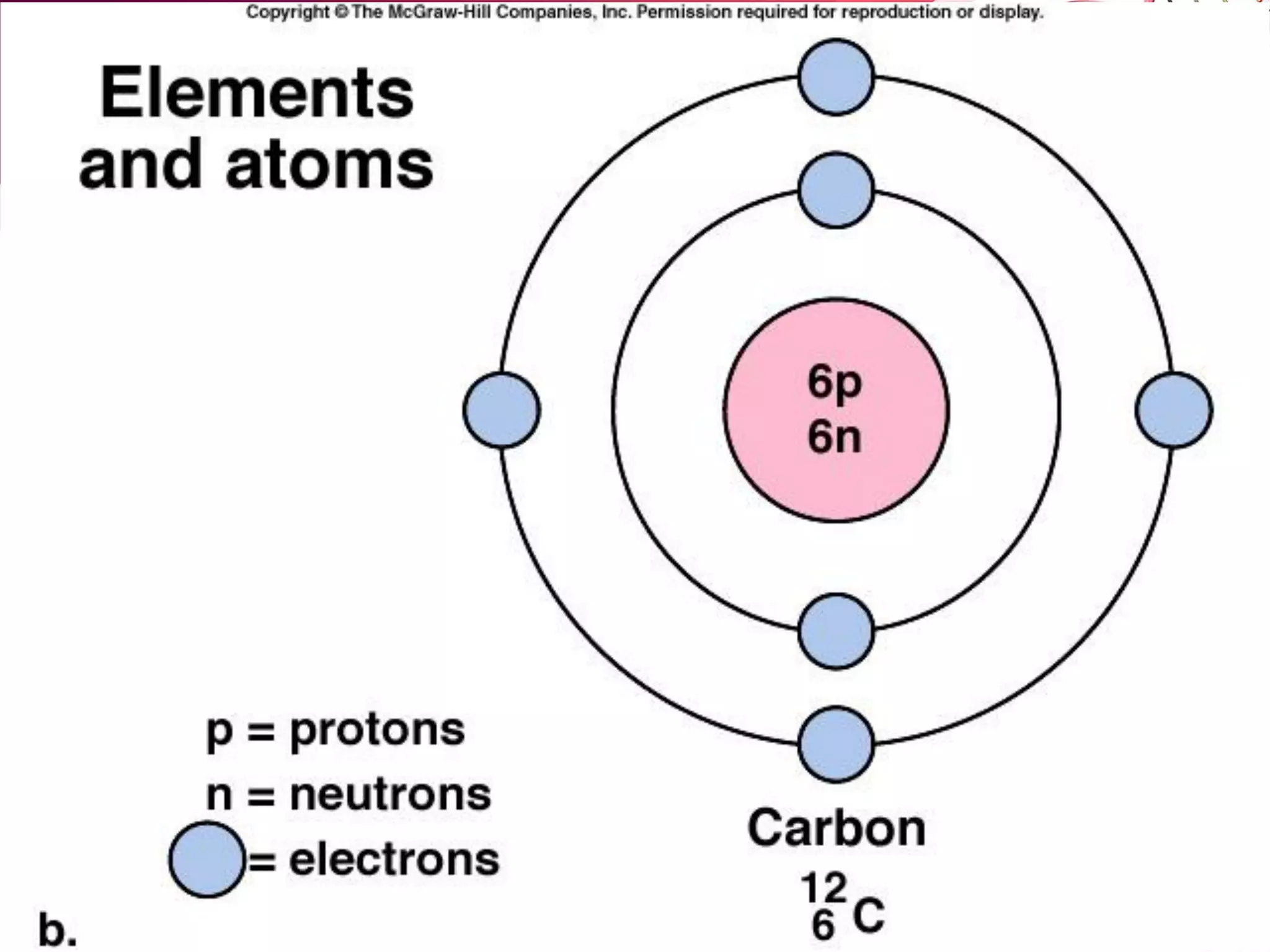
1. The document discusses the key chemical elements that make up the human body, including oxygen, carbon, hydrogen, nitrogen, calcium, and phosphorus.
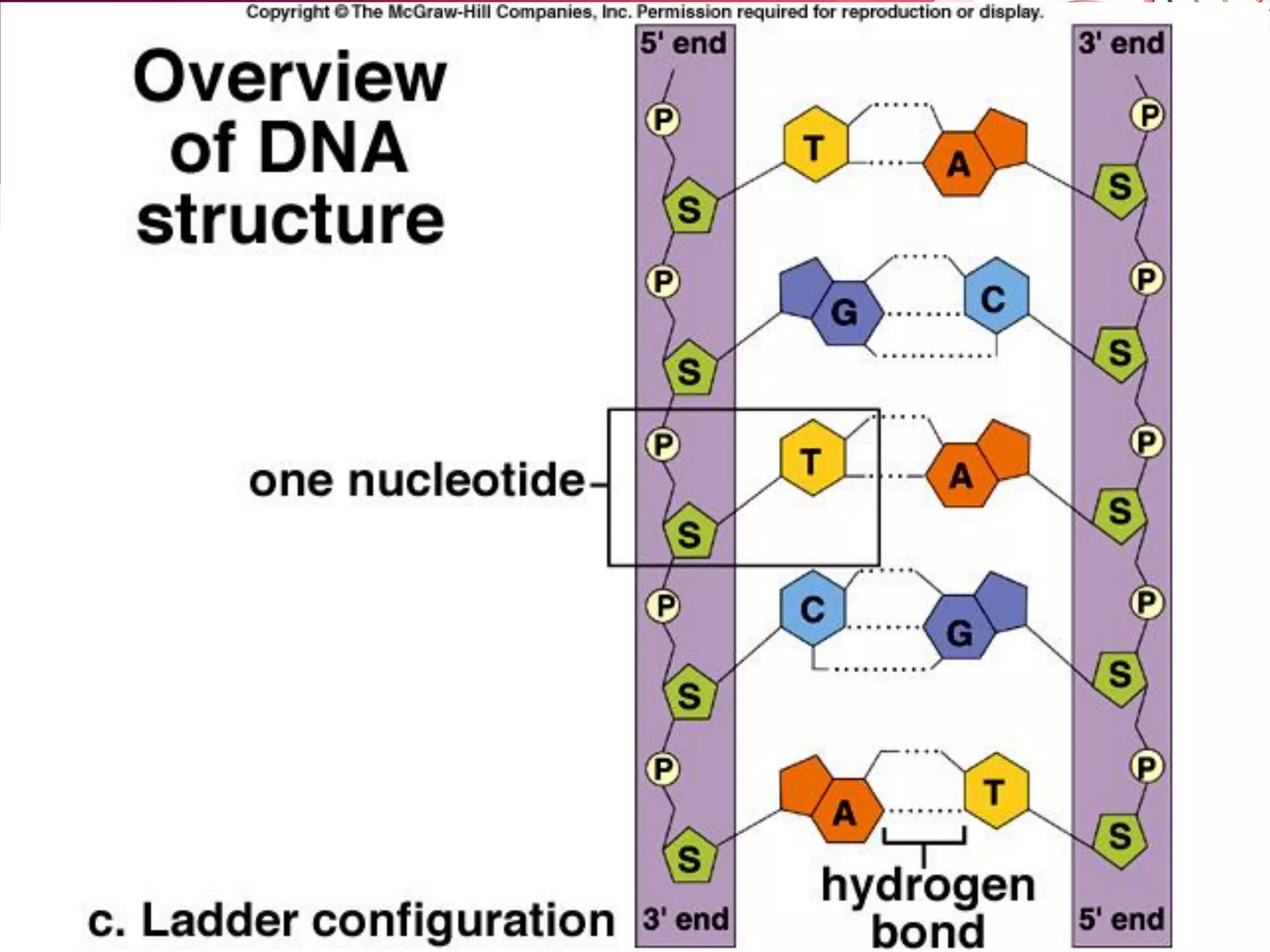
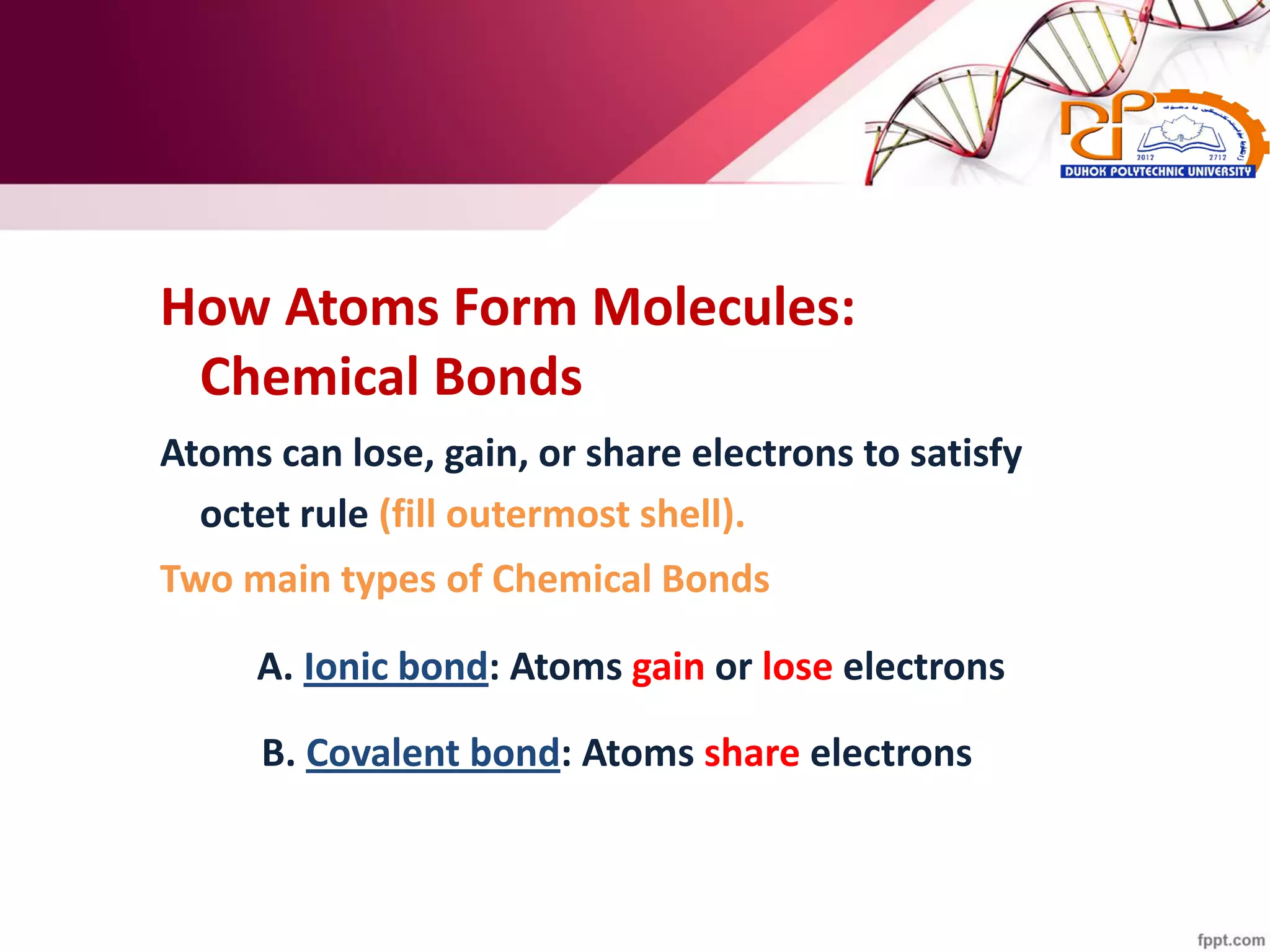
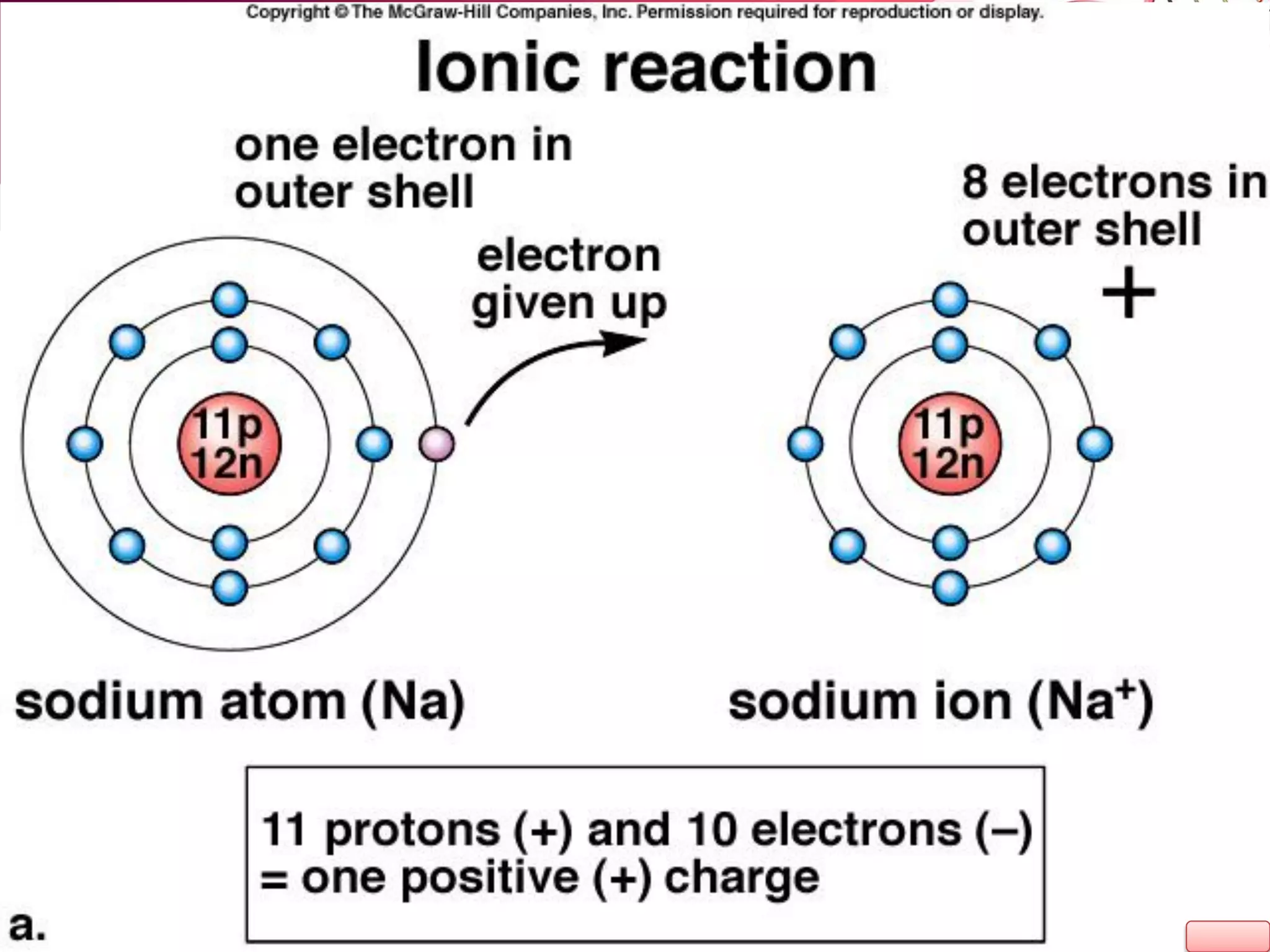
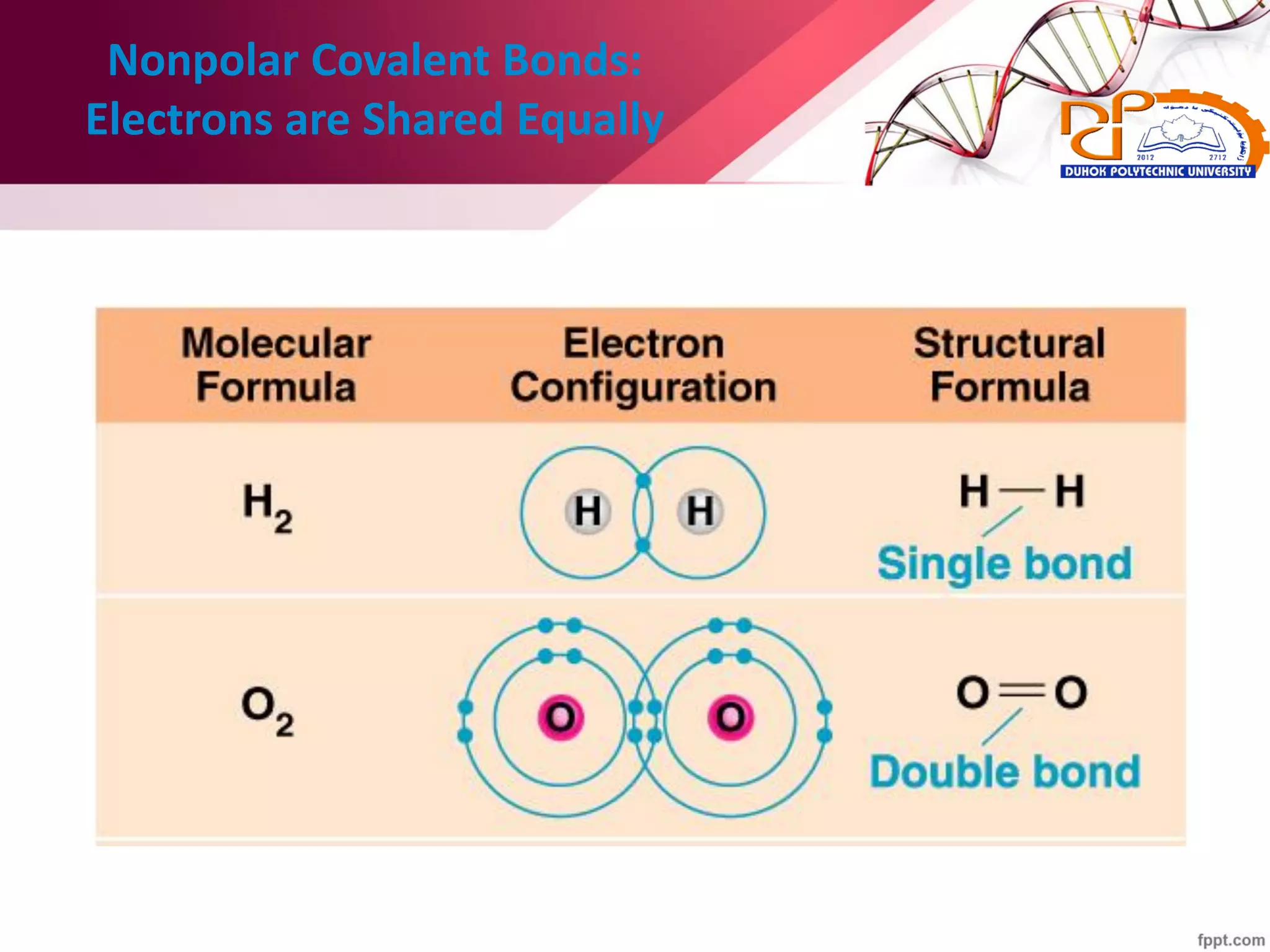
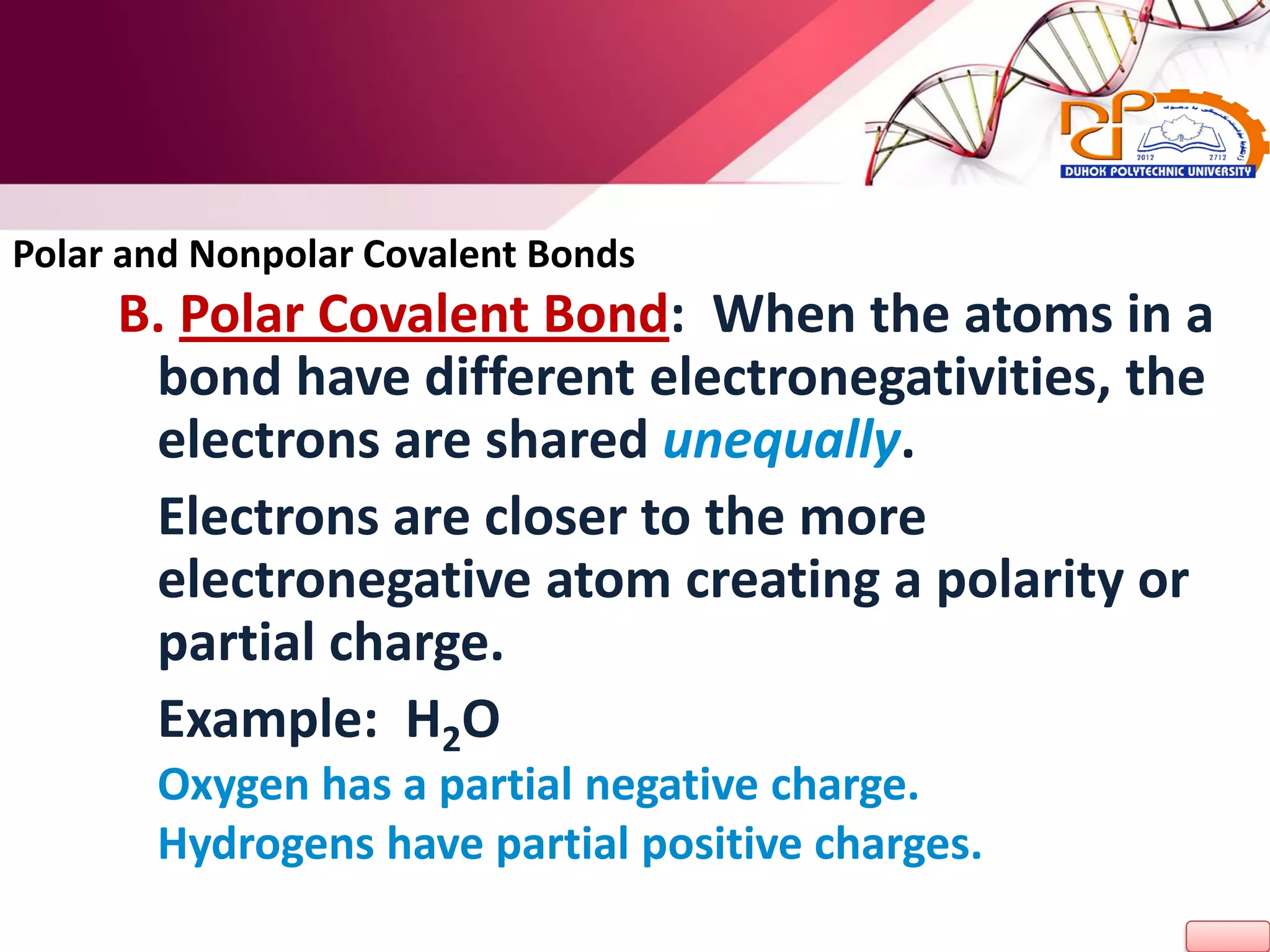
2. It also explains the structure of atoms and how they form bonds through ionic and covalent interactions. Water is highlighted as being an ideal solvent for life due to its unique properties arising from hydrogen bonding.
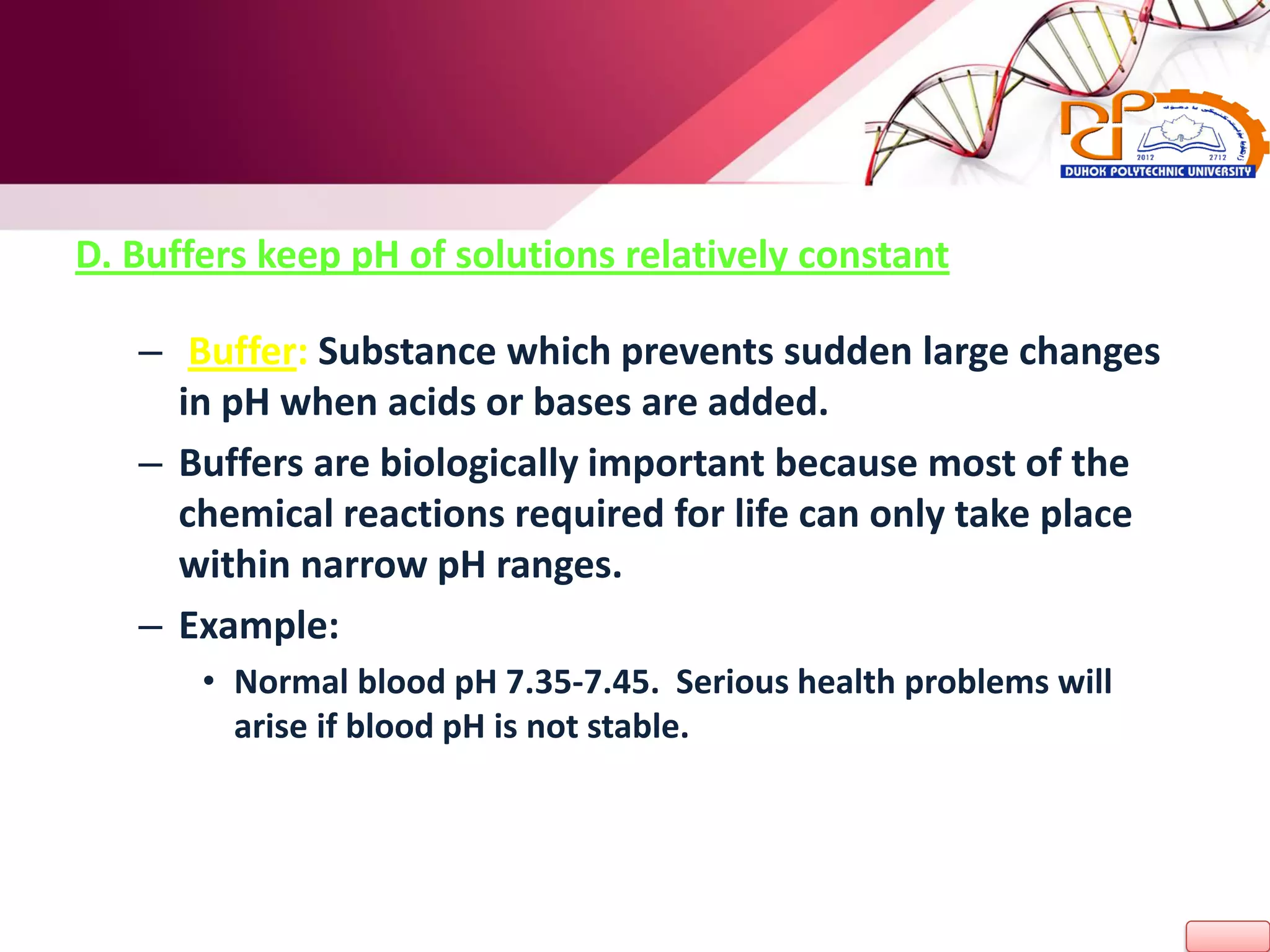
3. The roles of acids, bases, and pH in solutions are covered. Buffers are described as substances that help maintain a stable pH when acids or bases are introduced.
























![ACIDS, BASES, pH AND BUFFERS
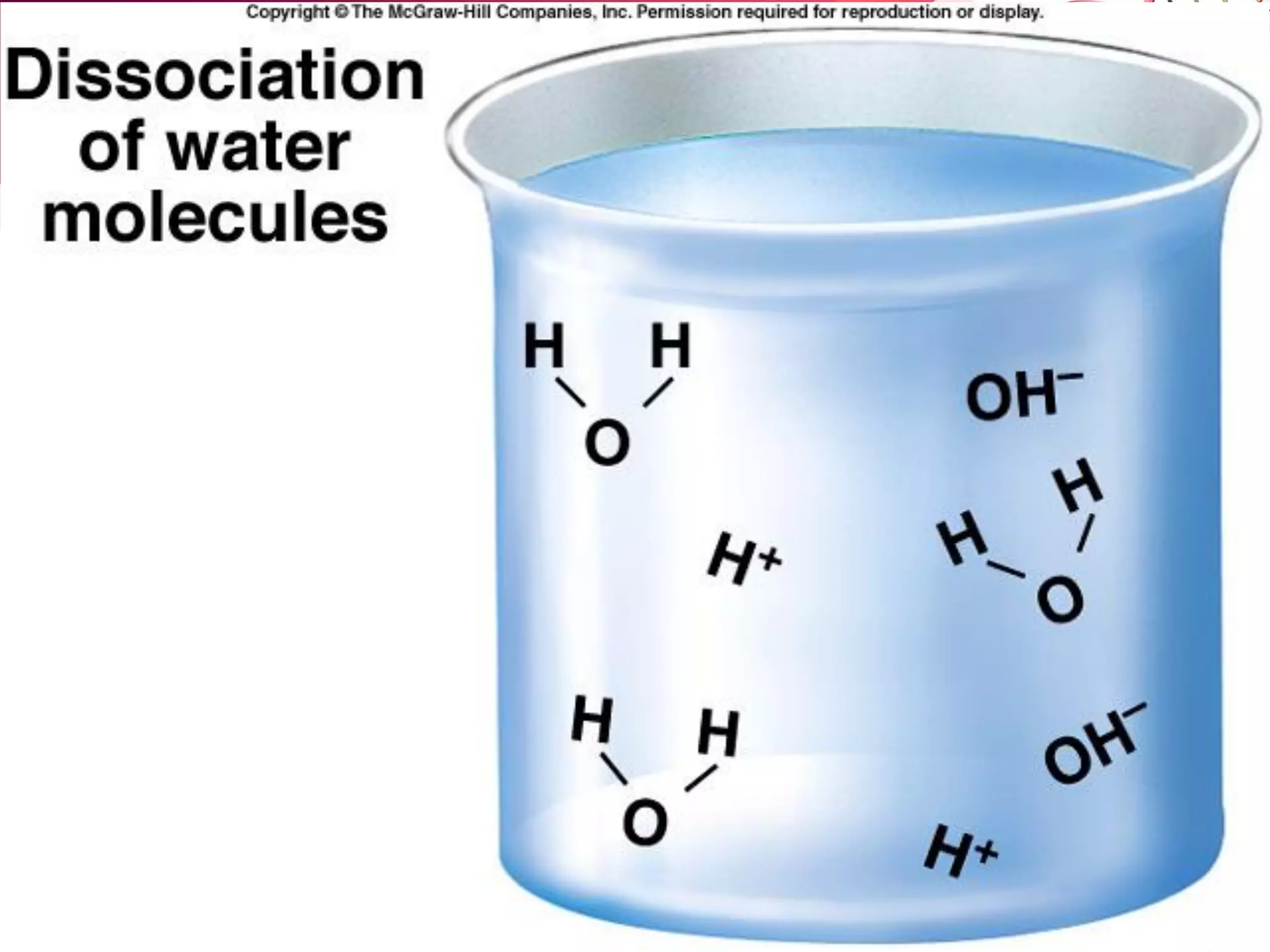
A. Acid: A substance that donates protons (H+).
– Separate into one or more protons and an anion:
HCl (into H2O ) -------> H+ + Cl-
H2SO4 (into H2O ) --------> H+ + HSO4
-
– Acids INCREASE the relative [H+] of a solution.
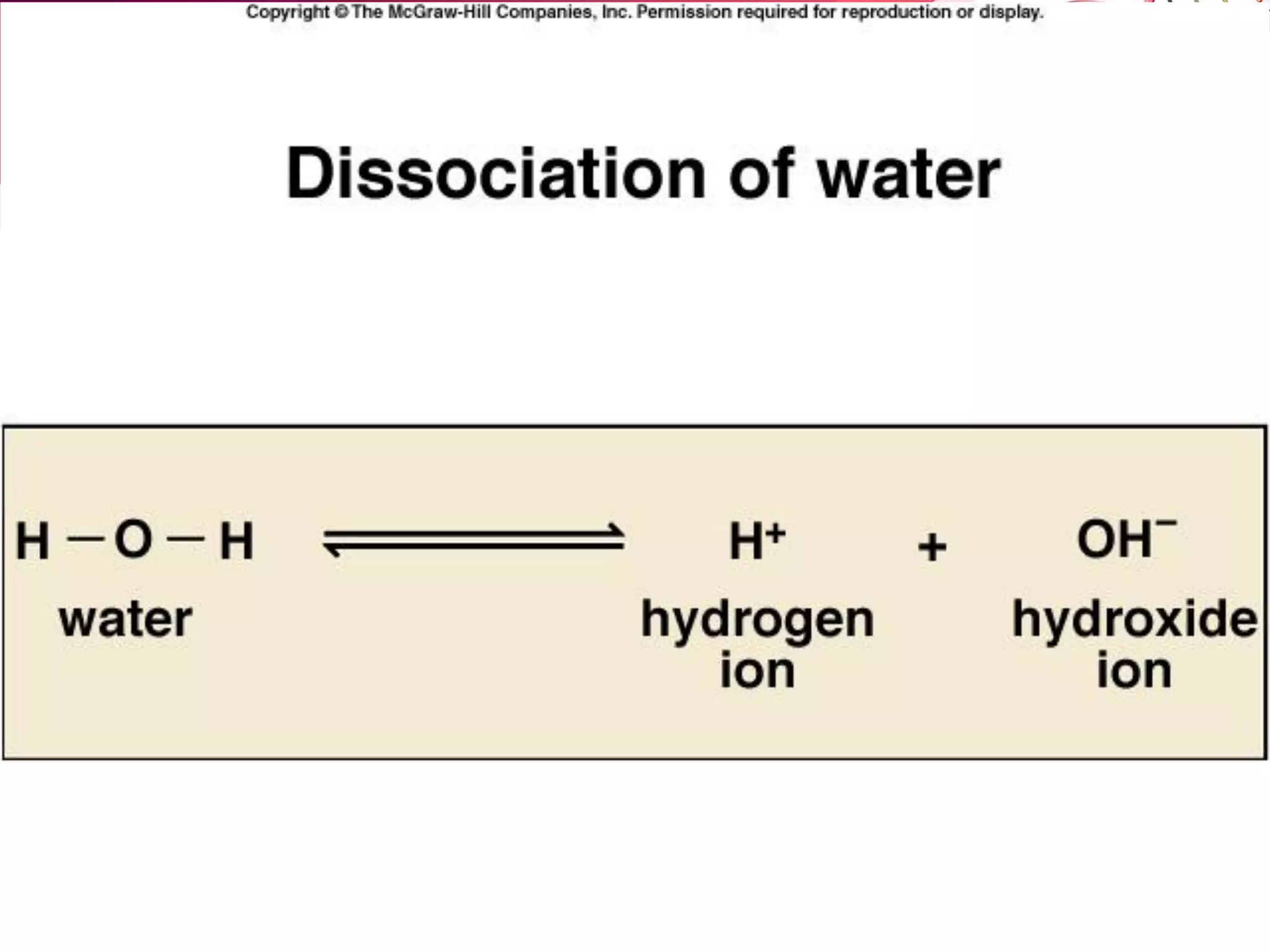
– Water can also dissociate into ions, at low levels:
H2O <======> H+ + OH-](https://image.slidesharecdn.com/l2-170107213131/75/L2-32-2048.jpg)
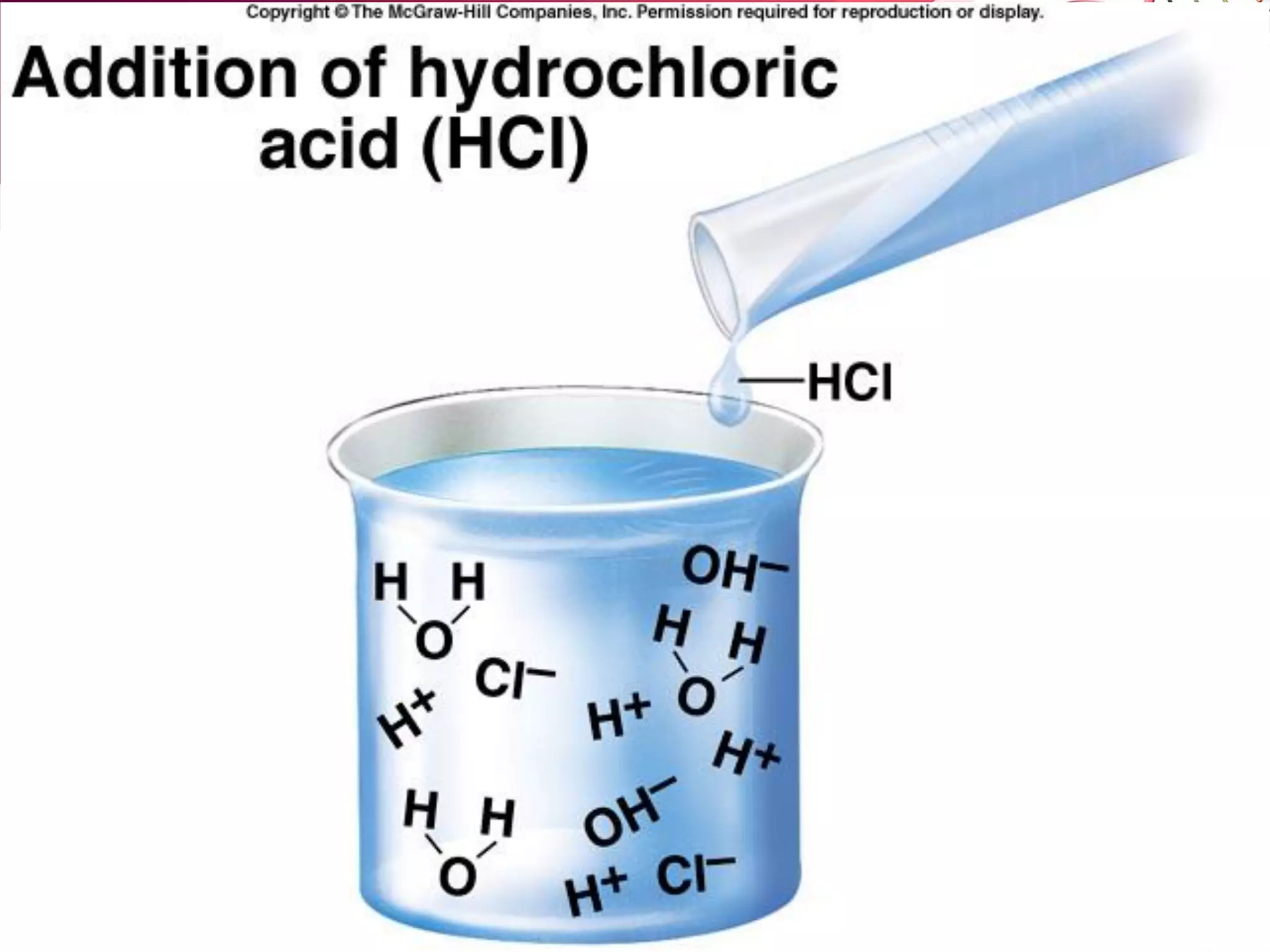
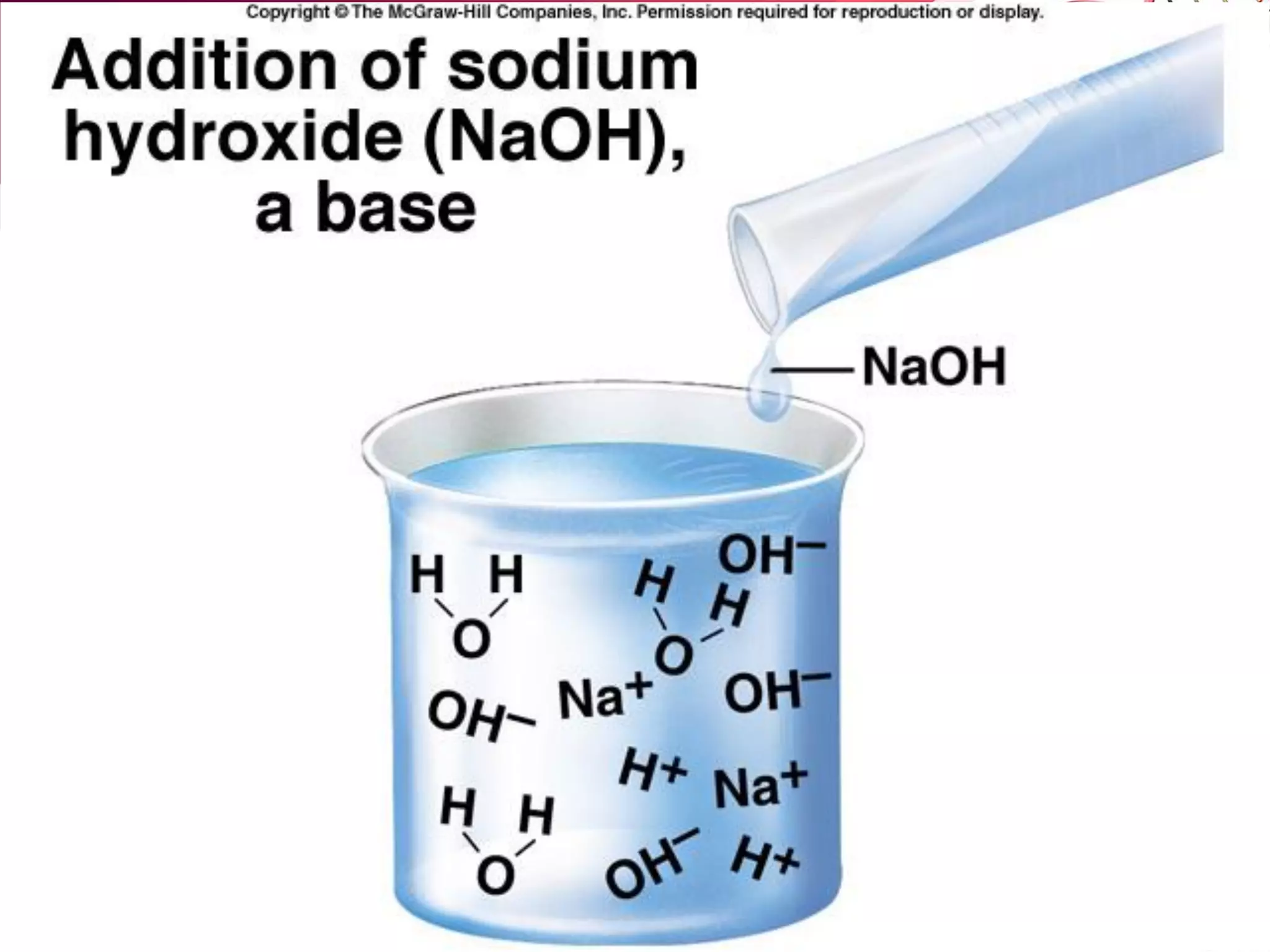
![B. Base: A substance that accepts protons (H+).
– Many bases separate into one or more positive ions
(cations) and a hydroxyl group (OH- ).
– Bases DECREASE the relative [H+] of a solution ( and
increases the relative [OH-] ).
H2O <======> H+ + OH-
Directly NH3 + H+ <=------> NH4
+
Indirectly NaOH ---------> Na+ + OH-
( H+ + OH- <=====> H2O )](https://image.slidesharecdn.com/l2-170107213131/75/L2-33-2048.jpg)

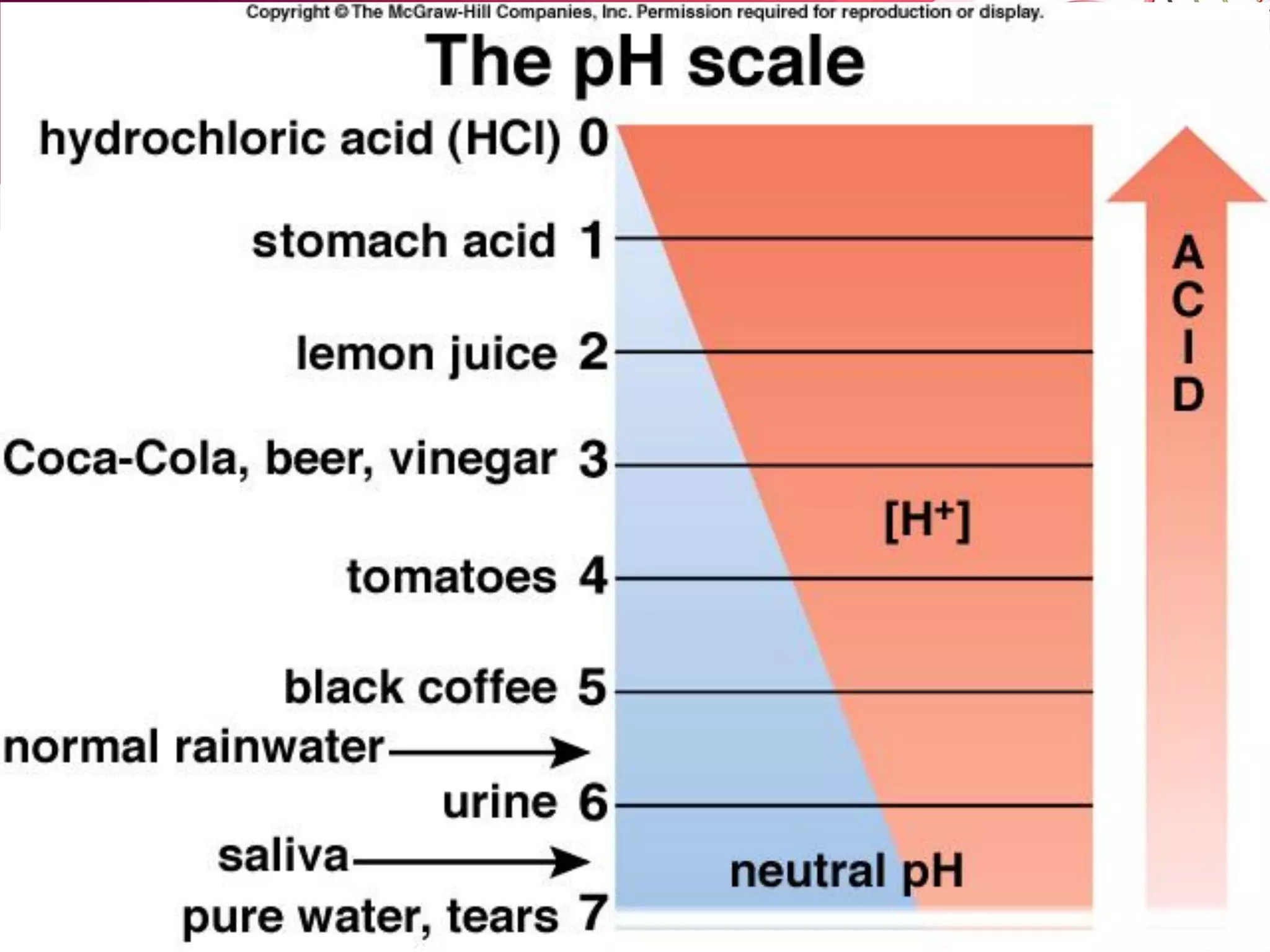
![C. pH scale: [H+] and [OH-]
– pH scale is used to measure how basic or acidic a solution
is.
– Range of pH scale: 0 through 14.
• Neutral solution: pH is 7. [H+ ] = [OH-]
• Acidic solution: pH is less than 7. [H+ ] > [OH-]
• Basic solution: pH is greater than 7. [H+ ] < [OH-]
– As [H+] increases pH decreases (inversely proportional).
– Logarithmic scale: Each unit on the pH scale represents a
ten-fold change in [H+].](https://image.slidesharecdn.com/l2-170107213131/75/L2-35-2048.jpg)