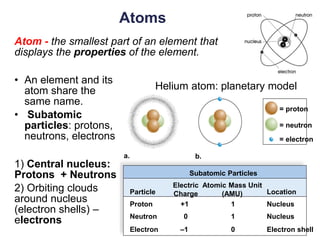
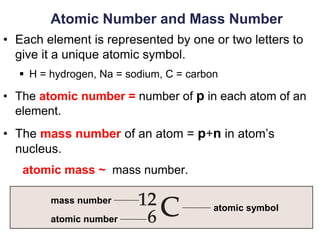
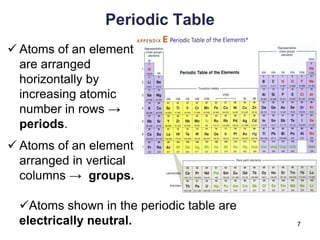
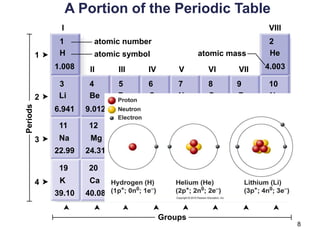
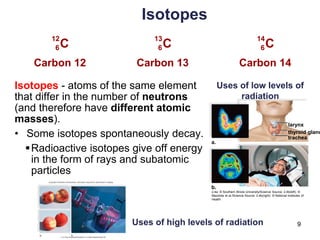
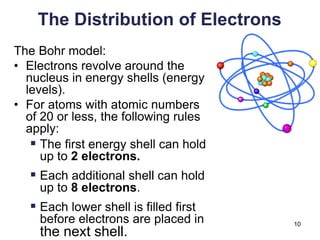
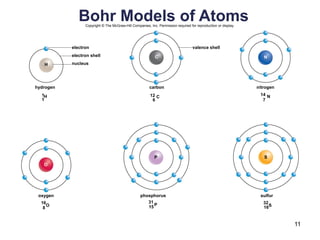
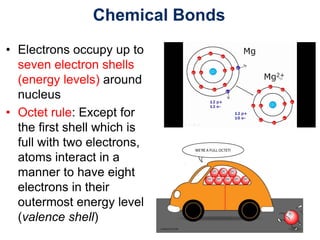
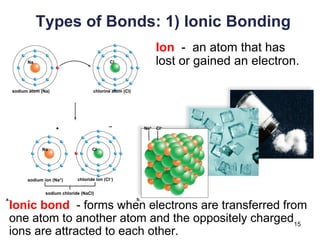
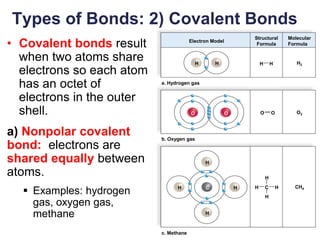
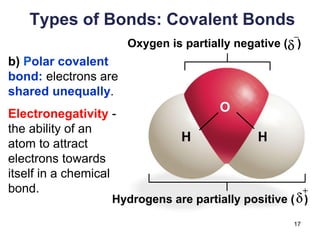
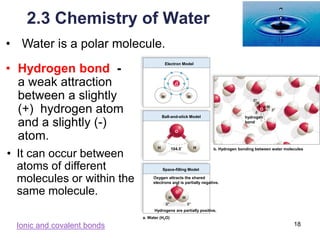
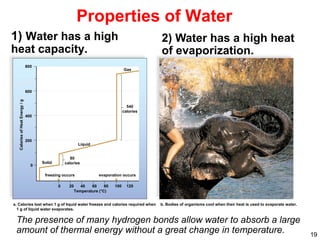
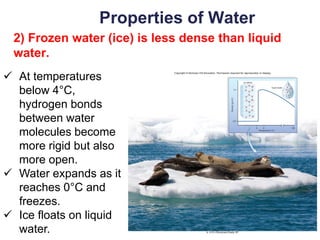
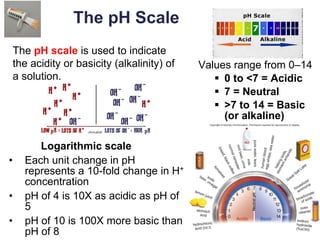
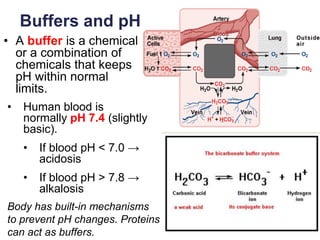
This document provides an overview of basic chemistry concepts. It defines elements and atoms, and explains that all matter is composed of elements. Atoms are made up of subatomic particles including protons, neutrons, and electrons. The periodic table arranges elements and displays their atomic numbers and masses. Molecules are formed via chemical bonds between elements, including ionic bonds and covalent bonds. Water is a polar molecule that has important properties like being a good solvent and existing in solid, liquid, and gas forms. Acids and bases are defined based on their ability to donate or accept hydrogen ions in water. The pH scale measures hydrogen ion concentration to indicate acidity or alkalinity. Buffers help maintain pH within a normal