Embed presentation
Downloaded 22 times




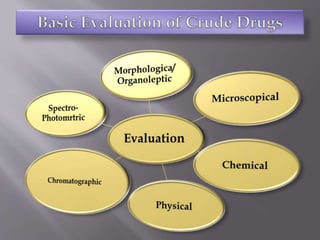


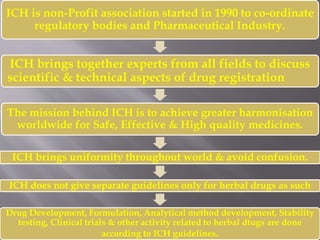






The document discusses international guidelines for herbal drugs. The International Council for Harmonization (ICH) brings together regulatory bodies and the pharmaceutical industry to achieve greater harmonization of standards for safe, effective, and high-quality medicines worldwide. While ICH does not have separate guidelines for herbal drugs, development, testing, and clinical trials of herbal medicines follow ICH standards. The World Health Organization also provides information and guidelines regarding traditional and herbal medicines, as over 60% of the world's population uses some form of traditional medicine.












