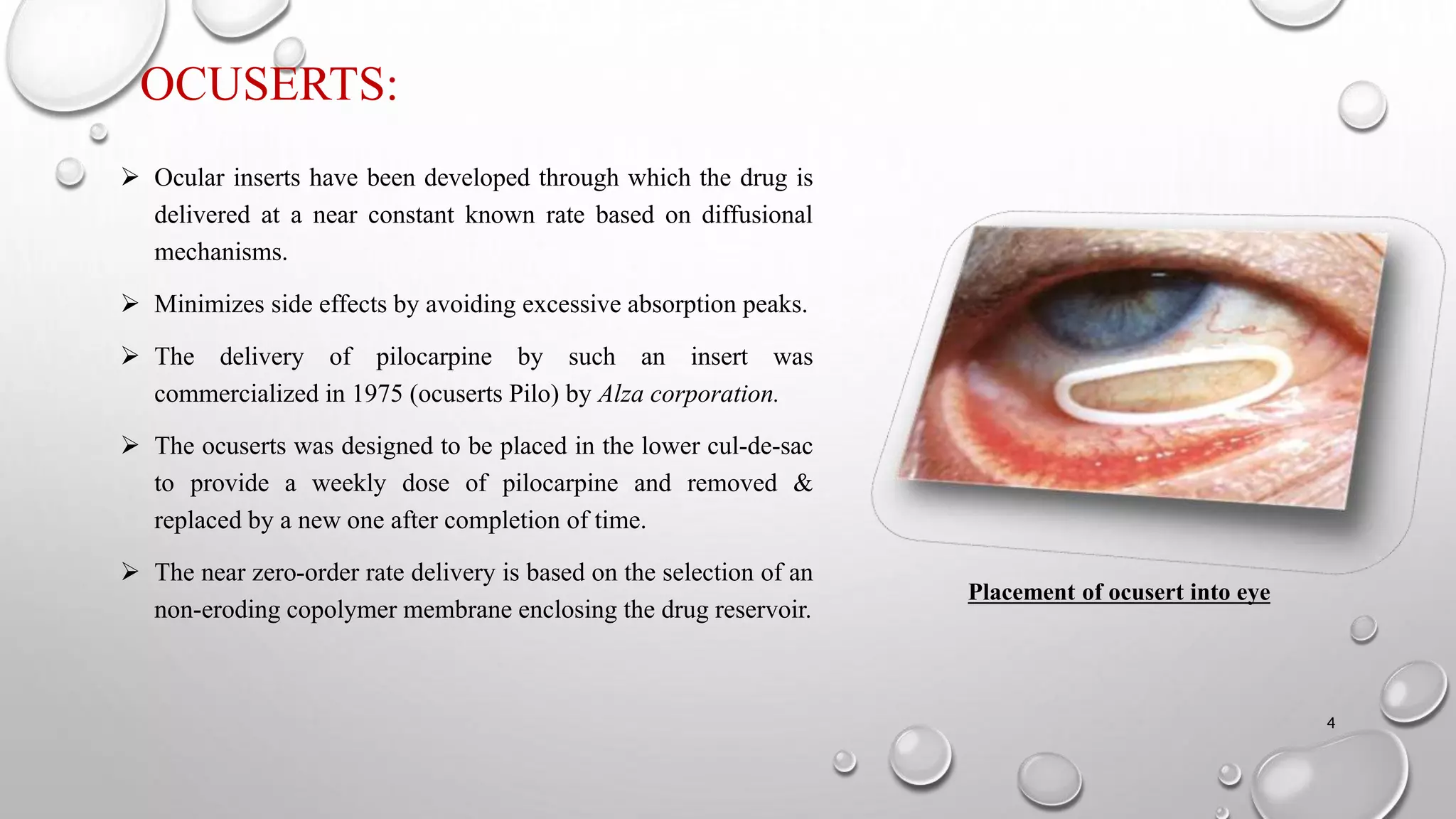
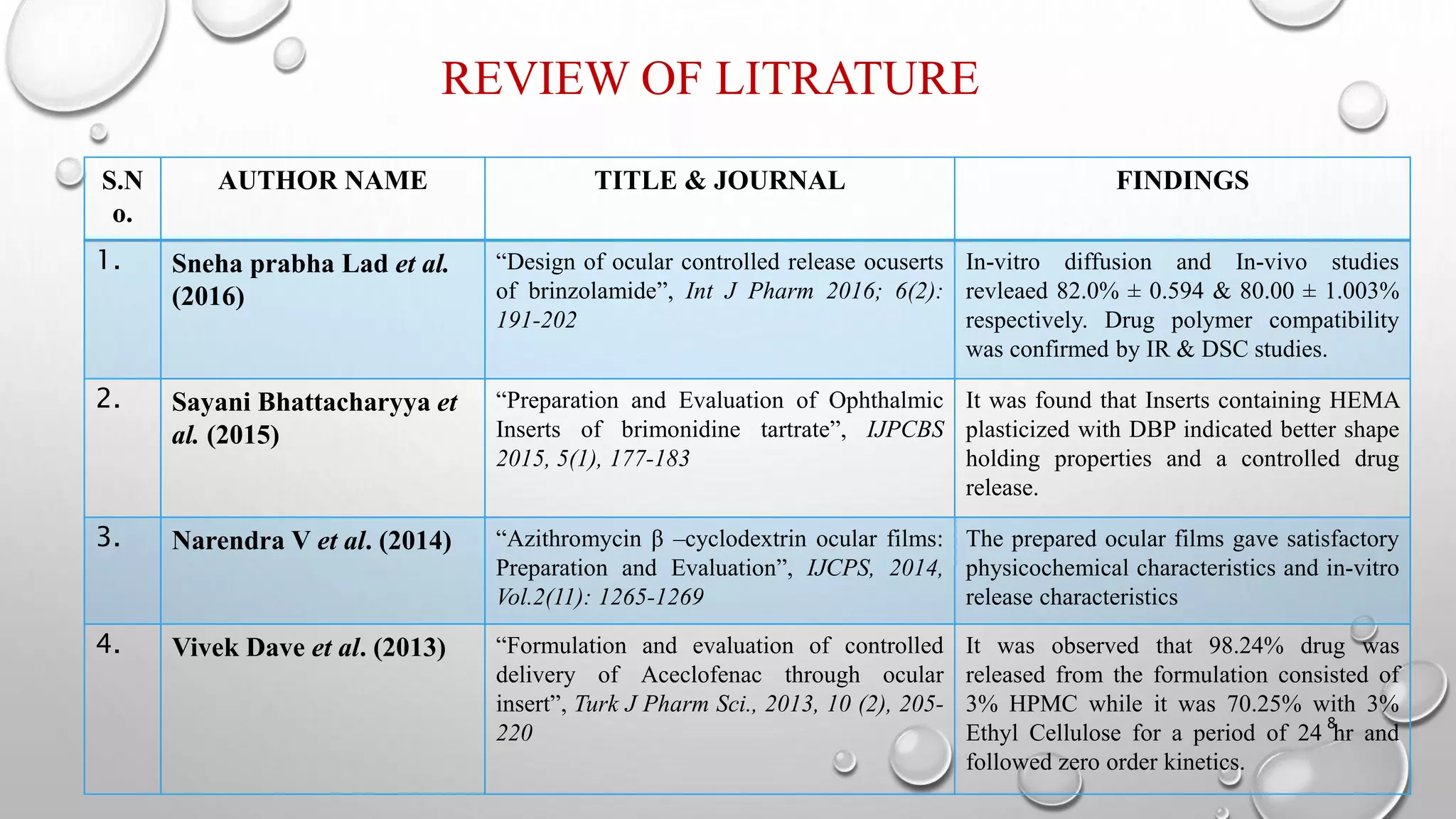
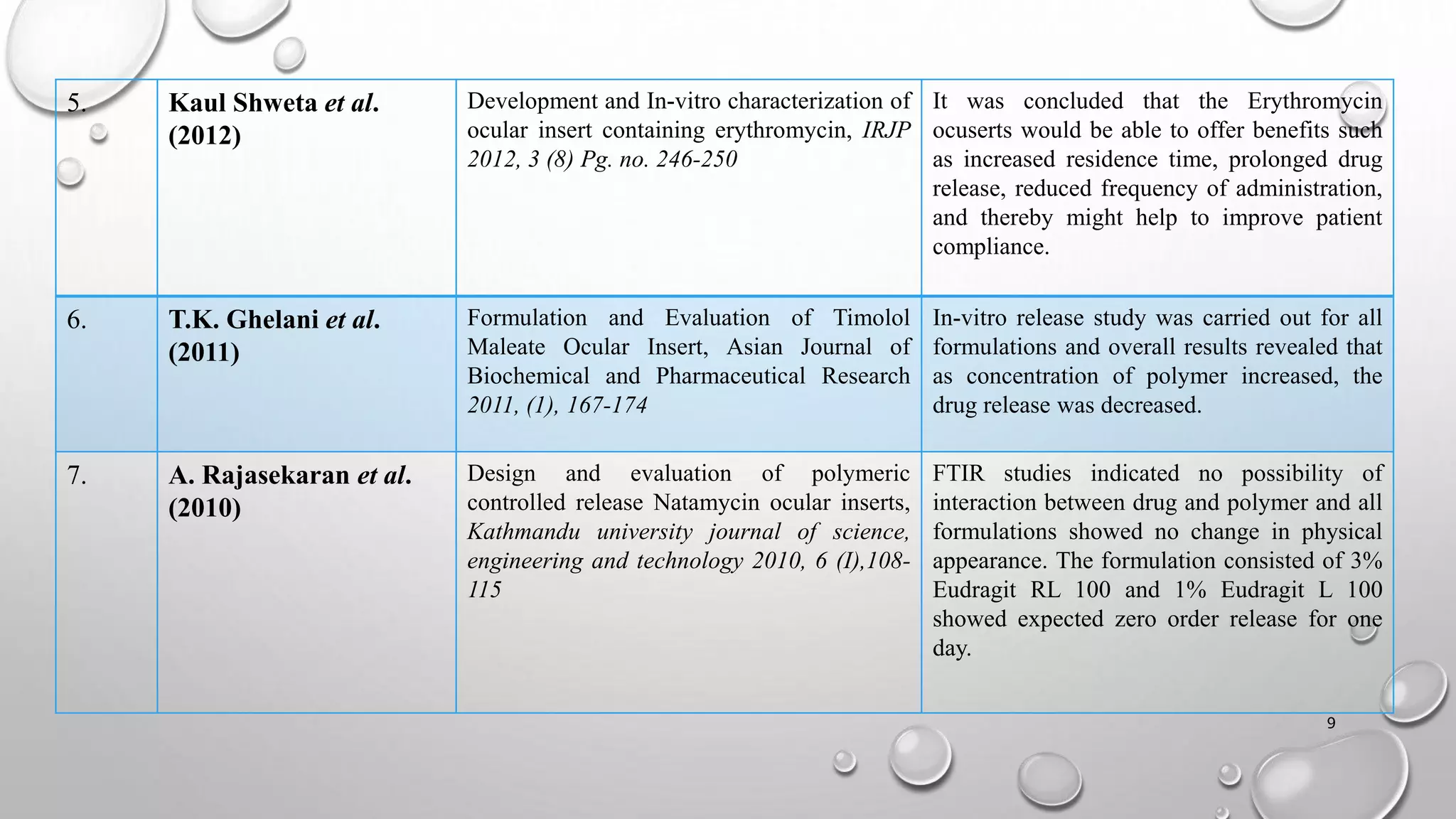
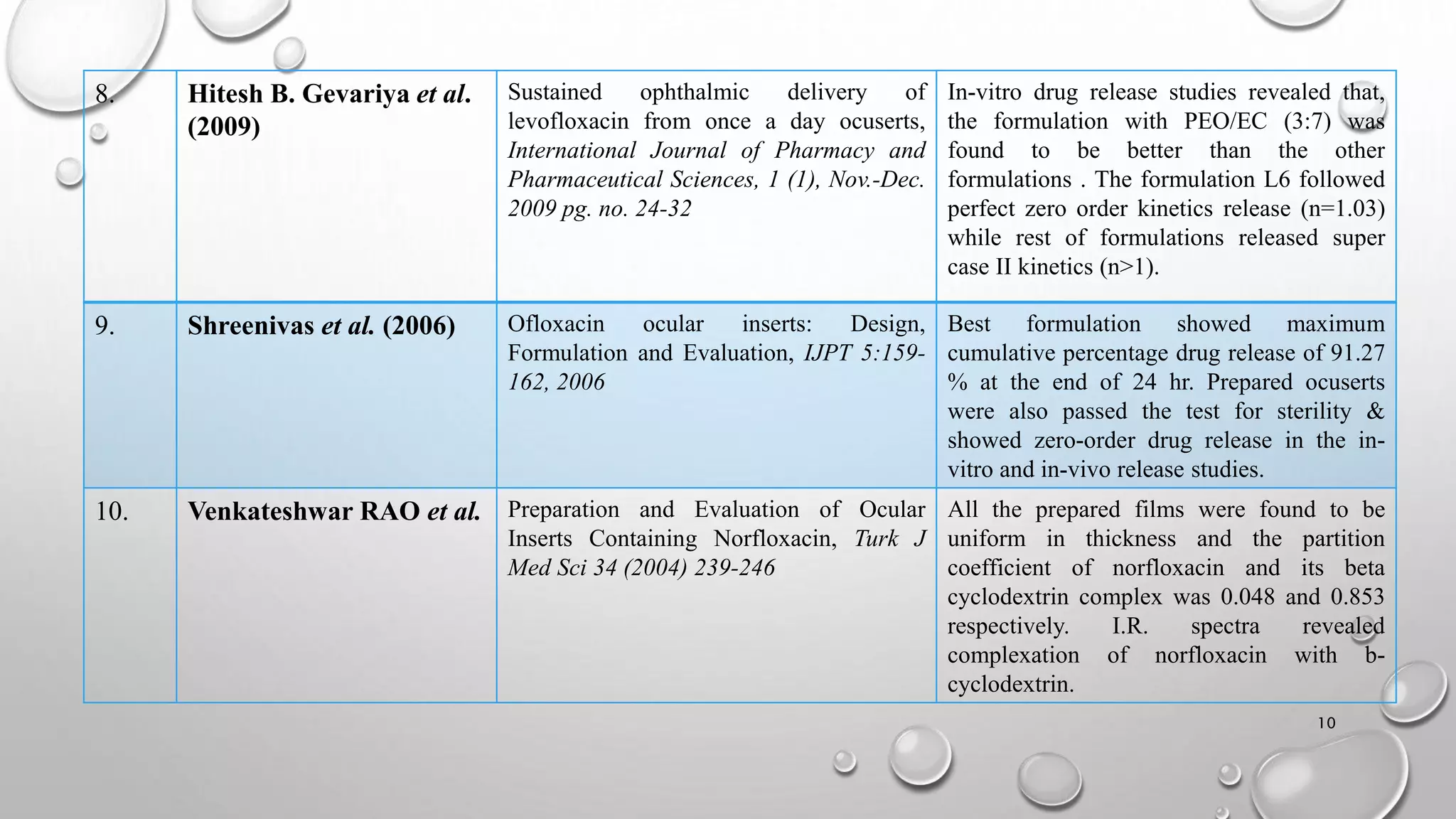
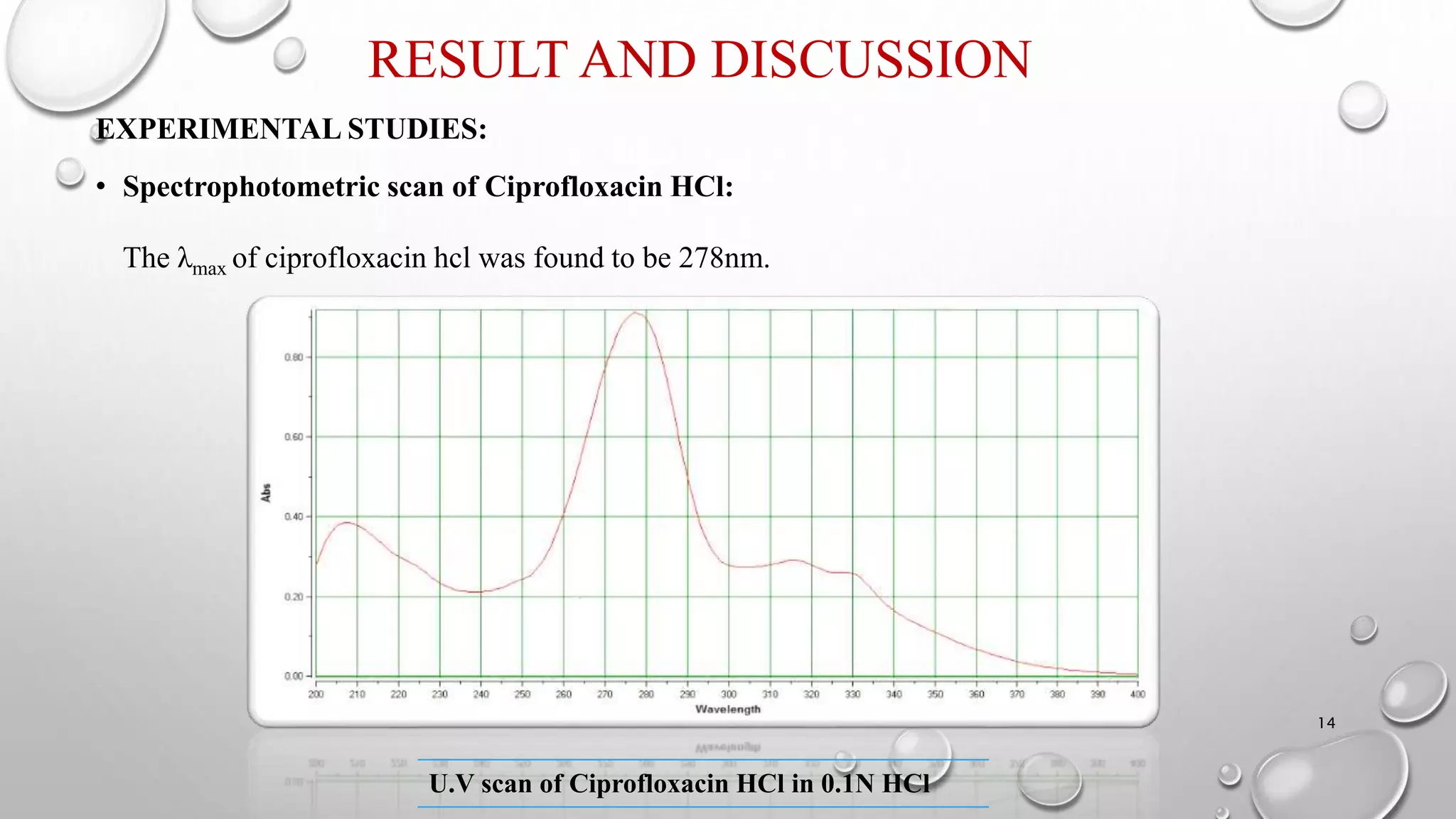
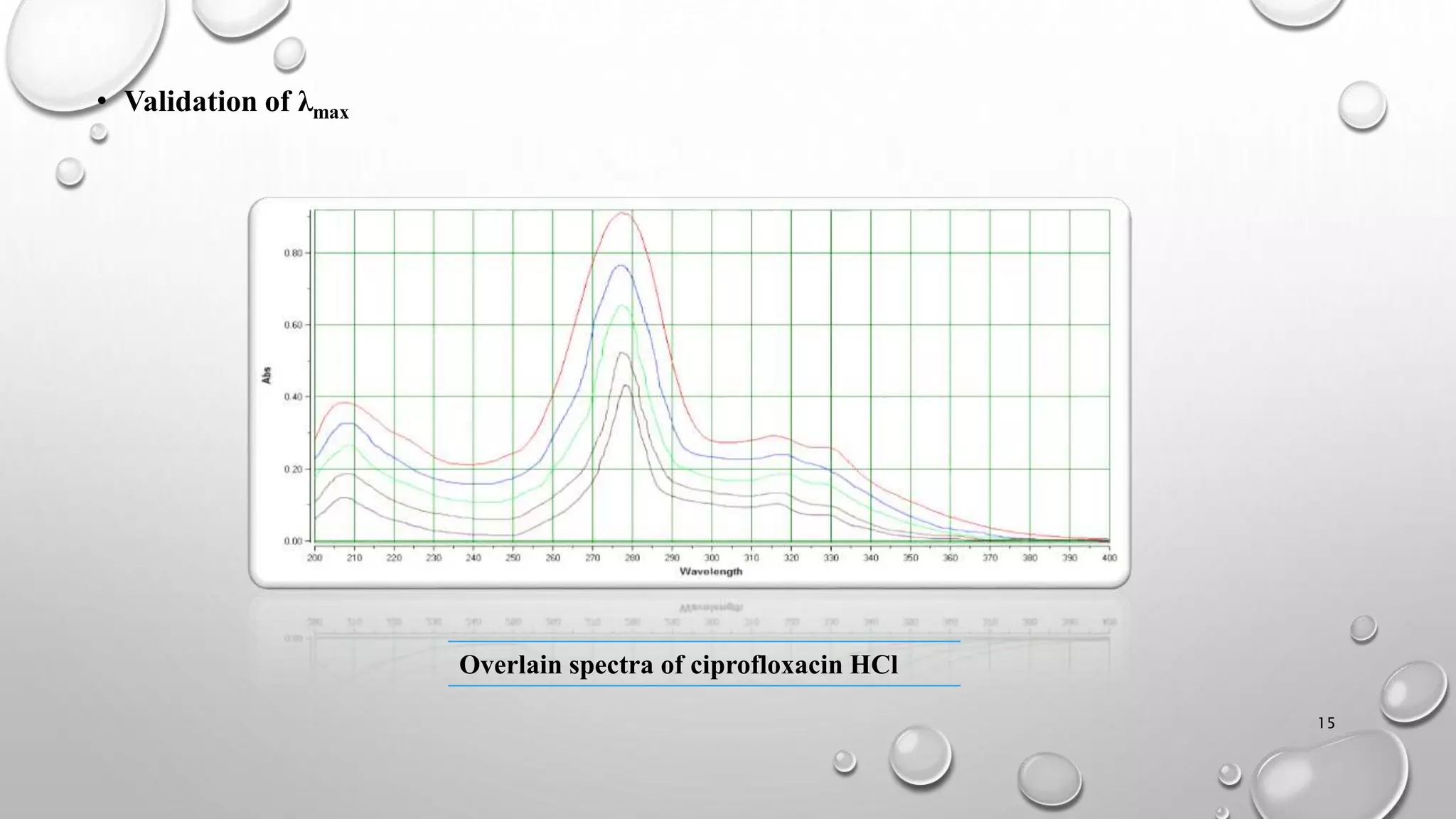
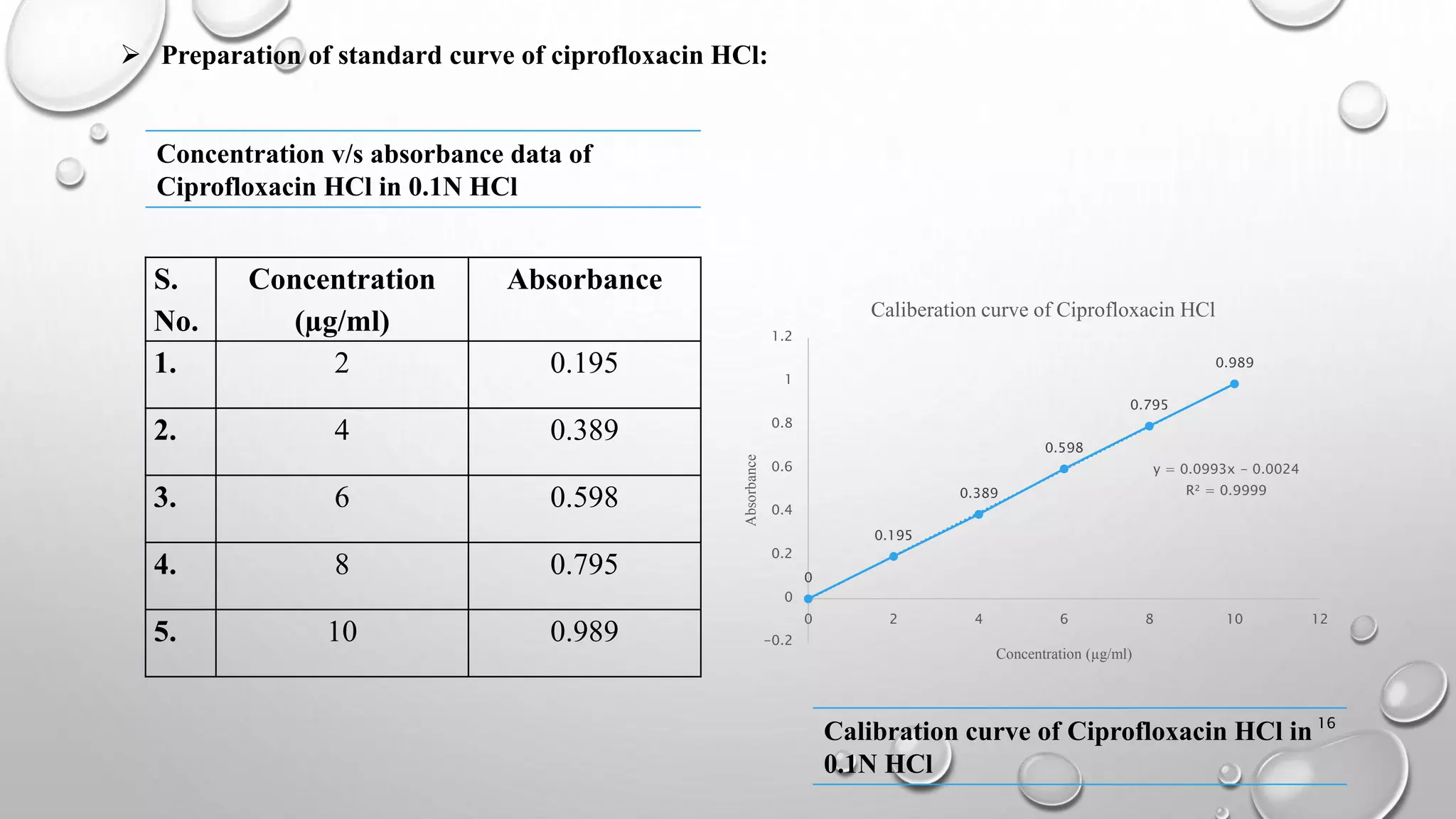
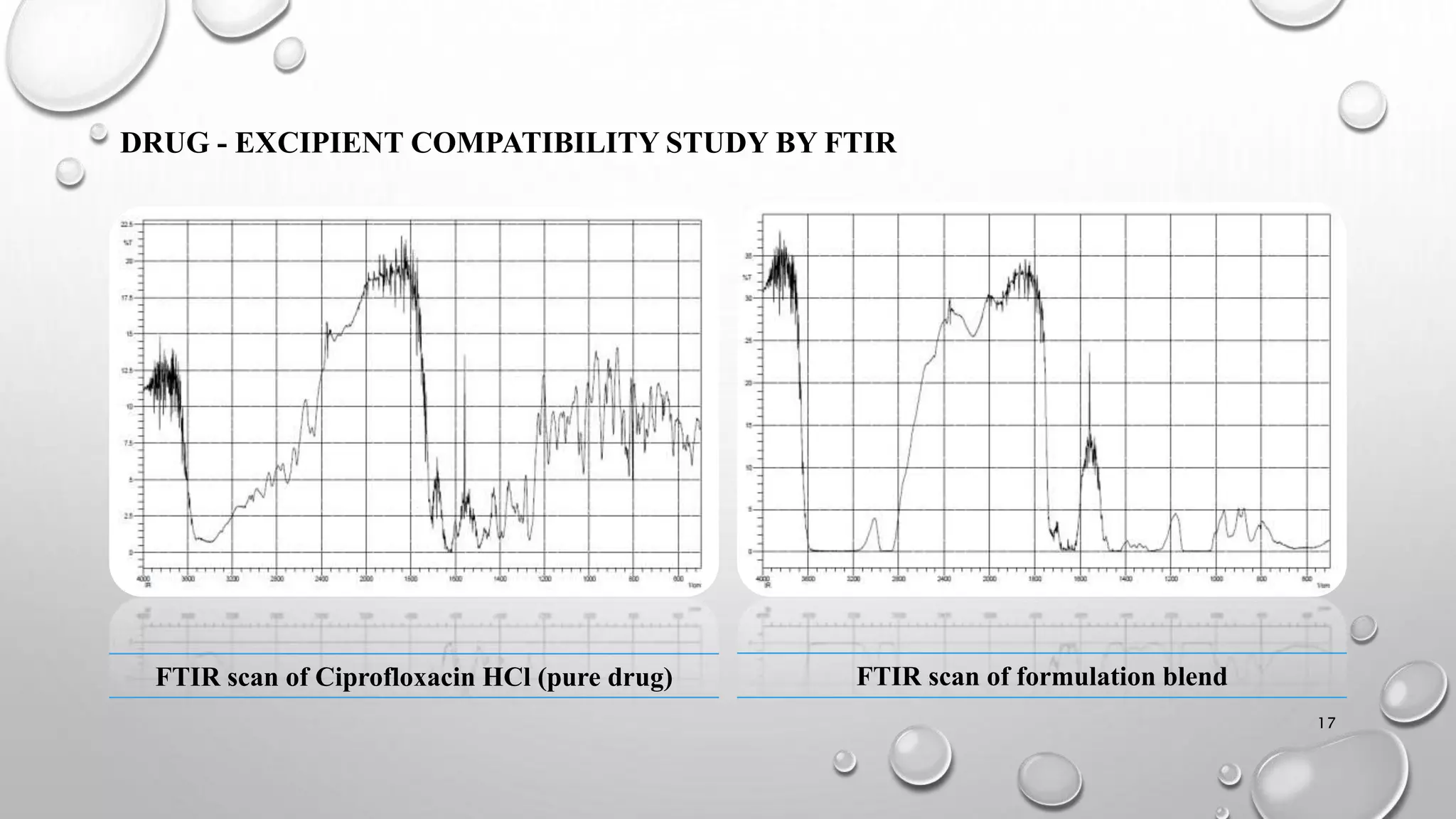
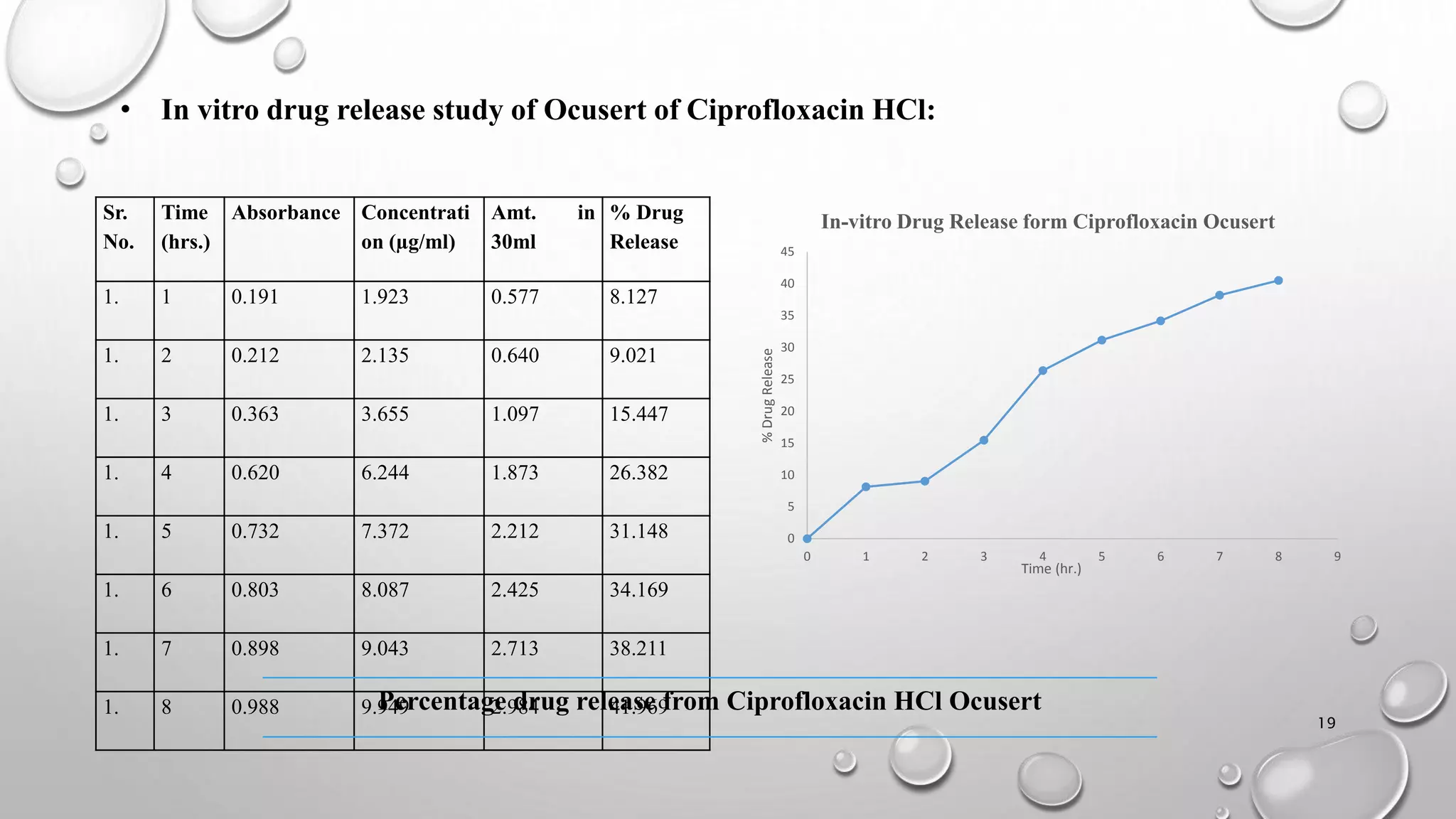
The document presents a study on the formulation and evaluation of ciprofloxacin HCl ocuserts, which are ocular inserts designed for sustained drug delivery. It describes the advantages of ocuserts over traditional ocular formulations, including prolonged drug release and improved patient compliance. The study includes methods of preparation, in vitro evaluation results, and concludes that the developed ocuserts could provide a better alternative for ocular drug delivery.