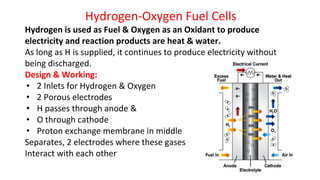
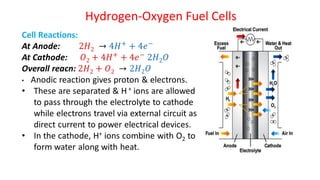
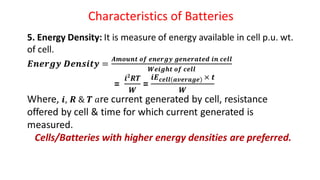
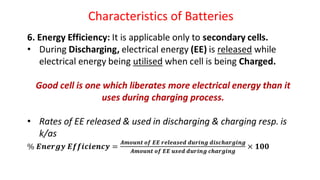
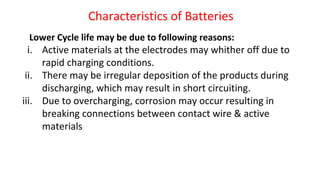
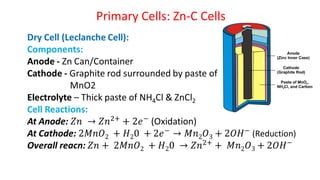
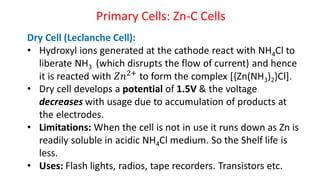
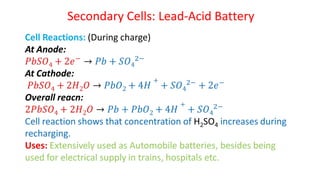
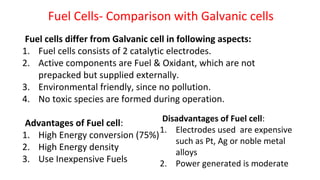
Batteries are electrochemical devices that convert chemical energy into electrical energy. They consist of one or more electrochemical cells with external connections to power devices. Batteries can be primary cells, which are not rechargeable, or secondary cells, which are rechargeable. Common battery types include zinc-carbon batteries, alkaline batteries, lead-acid batteries, lithium-ion batteries, and hydrogen-oxygen fuel cells. Fuel cells directly convert chemical energy from a fuel into electricity through redox reactions, providing higher efficiencies than conventional energy generation methods.

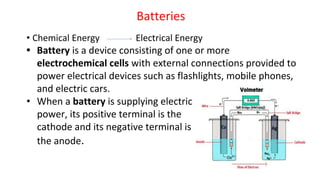
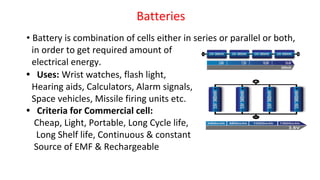



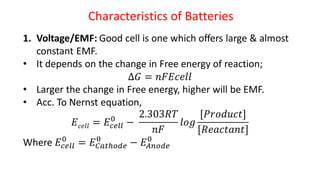
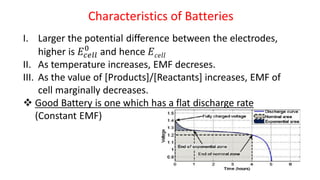
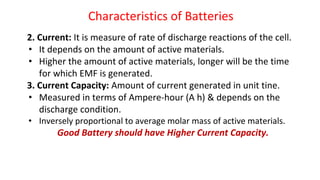
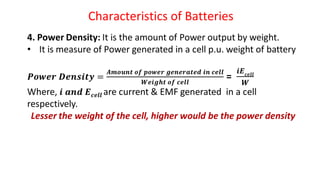












![Fuel Cells
Cell Representation:
Fuel Electrode [A] Electrolyte Electrode [C] Oxidant
Chemical reactions involved at electrodes:
At Anode: Fuel Oxidation product + ne-
At Cathode: Oxidant + ne-
Reduction product
Overall: Fuel + Oxidant Oxidn product + Redn product
Frequently used fuels are hydrogen, methanol, ethanol, hydrazine,
formaldehyde, Carbon monoxide & Alkanes.
Oxidant is pure Oxygen](https://image.slidesharecdn.com/battery-231116215704-138c4970/85/battery-pdf-30-320.jpg)