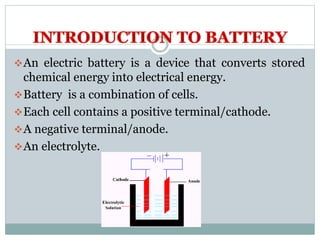
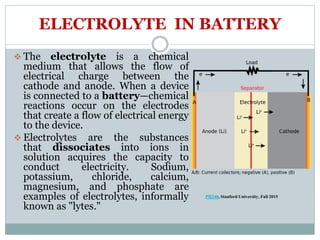
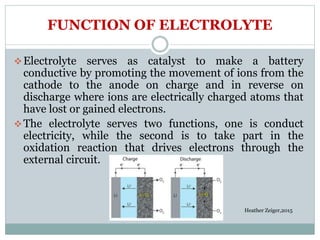
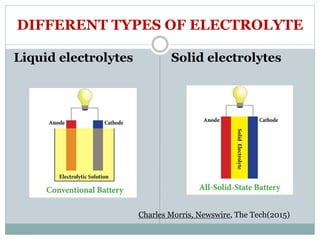
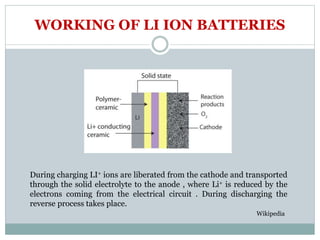
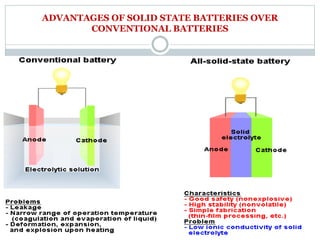
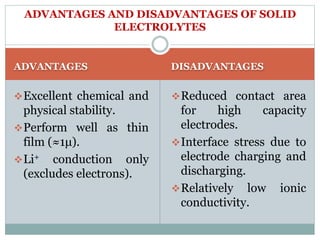
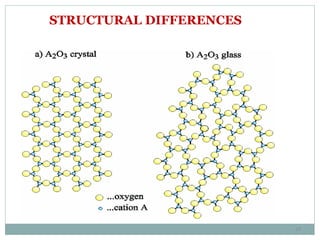
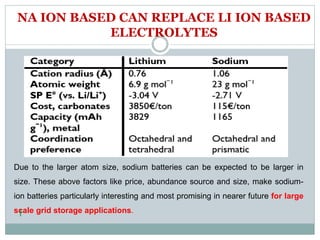
This document presents a seminar on solid electrolytes for next-generation batteries, discussing the role, advantages, and disadvantages of both liquid and solid electrolytes. It emphasizes the progression from liquid to solid electrolytes due to issues like leakage and dendritic growth, highlighting sodium-ion batteries as a promising alternative to lithium-ion batteries due to their cost-effectiveness and abundance. The document concludes by noting the significance of electrolytes in battery function and the ongoing research into materials suitable for future battery technologies.