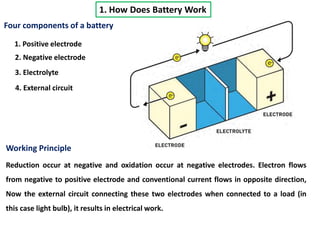
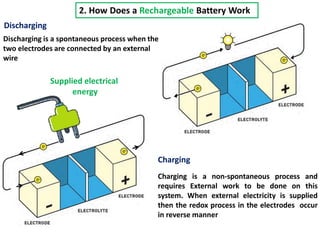
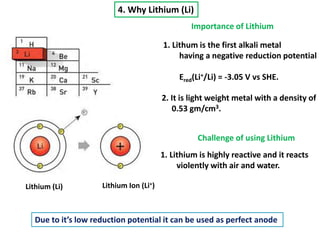
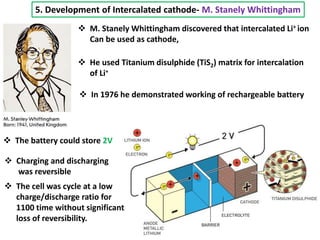
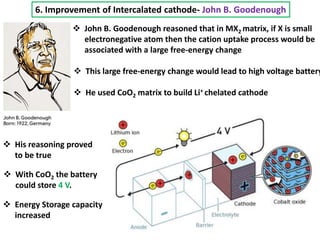
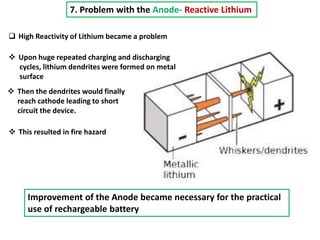
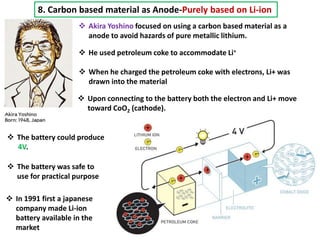
The document summarizes the development of the lithium-ion battery, which was awarded the 2019 Nobel Prize in Chemistry. It describes the key contributions of M. Stanley Whittingham, who developed the first lithium battery in 1976 using titanium disulfide as the cathode; John B. Goodenough, who doubled the battery's voltage to 4 volts using a cobalt oxide cathode in 1980; and Akira Yoshino, who created the first commercially viable lithium-ion battery in 1991 by replacing the lithium anode with a carbon-based material. The document also shares comments from 97-year-old John Goodenough upon receiving the Nobel Prize, emphasizing the importance of curiosity, hard work, and continuing to think every