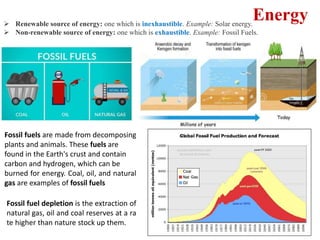
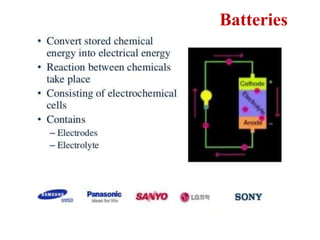
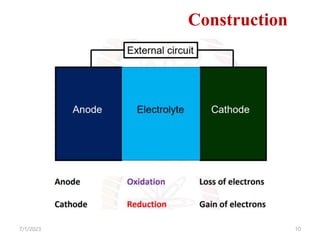
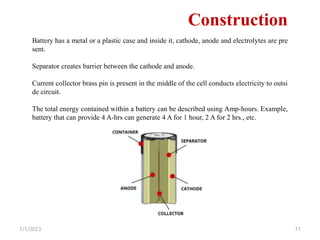
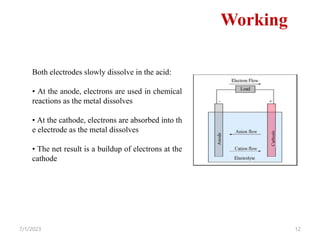
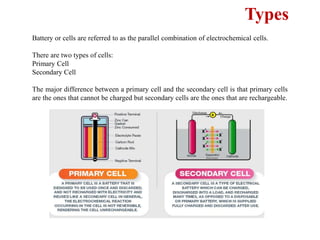
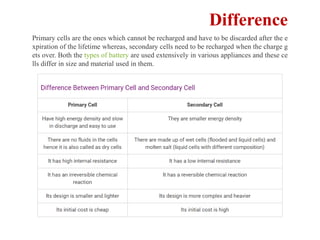
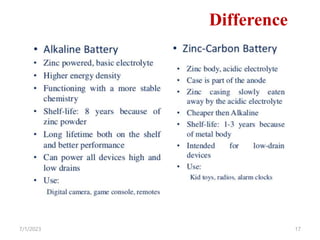
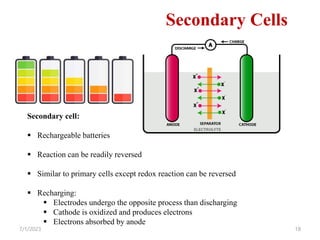
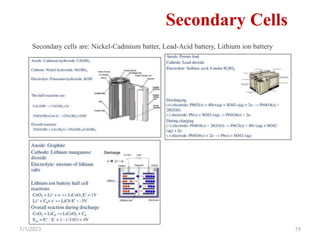
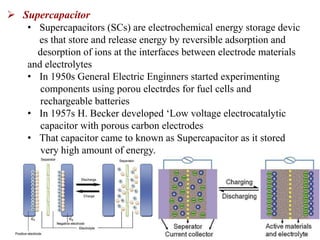
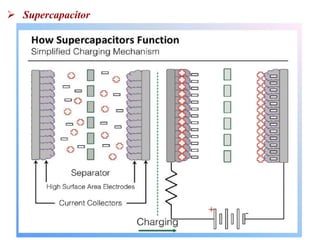
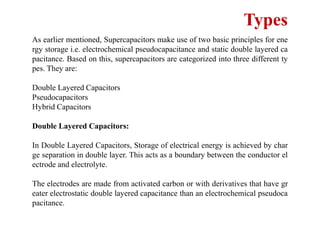
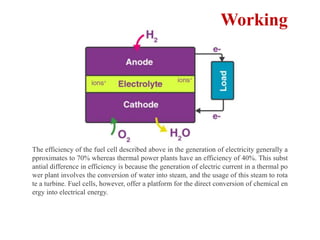
This document provides an overview of energy storage and conversion devices including batteries, fuel cells, and supercapacitors. It discusses the principles and components of these devices, as well as their applications. Specifically, it describes the basic structure and workings of batteries, fuel cells, and supercapacitors. It also covers the different types of each device and their advantages and limitations.