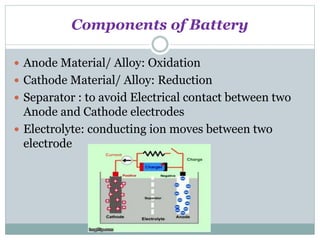
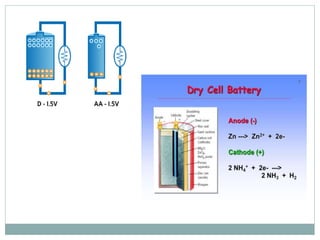
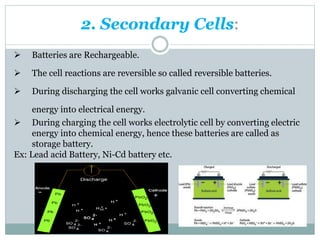
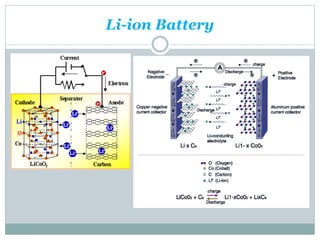
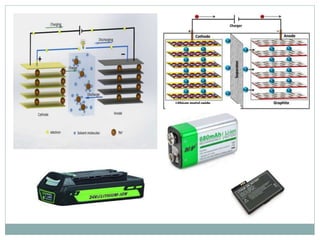
This document discusses different types of battery technology. It introduces batteries and their components. There are three main types of batteries: primary cells which are not rechargeable, secondary cells which are rechargeable, and reserve batteries which have a long shelf life. Two specific battery technologies discussed are nickel-metal hydride batteries, which use metal hydrides as the anode, and lithium-ion batteries. Key characteristics of batteries include their emf, current, capacity, efficiency, cycle life, power density, and shelf life.