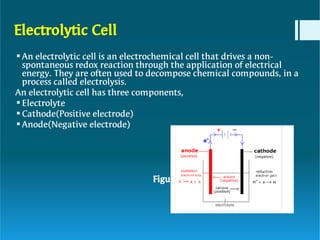
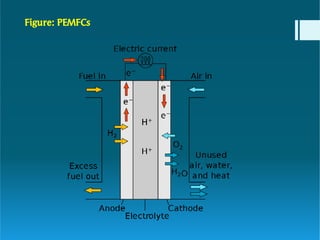
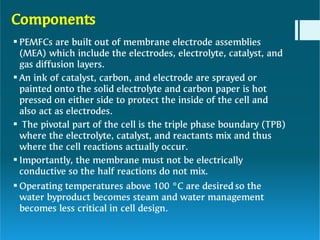
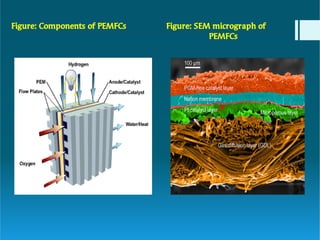
The document discusses polymer electrolytes and their applications in electrochemical devices, particularly in fuel cells. It details the types of electrolytic cells, polymer electrolytes, biopolymer electrolytes, their properties, and the importance of dopants for conductivity. Additionally, it covers the principles of polymer electrolyte membrane fuel cells, their efficiency, construction, and potential applications in transportation and energy generation.

















![Efficiency
The maximal theoretical efficiency applying the Gibbs free
energy equation ΔG = −237.13 kJ/mol and using the heating
value of Hydrogen (ΔH = −285.84 kJ/mol) is 83% at 298 K.
η = Δ G /Δ H = 1 − [T Δ S /Δ H]
The practical efficiency of a PEMs is in the range of 50–60% .
Main factors that create losses are:
Activation losses
Ohmic losses
Mass transport losses](https://image.slidesharecdn.com/polymerelectrolytesandfuelcells-200314162126/85/Polymer-electrolytes-and-fuel-cells-22-320.jpg)

