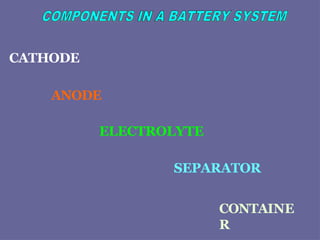
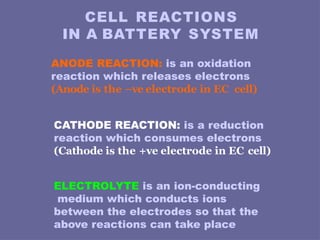
The document discusses the principles and classifications of batteries as energy sources, detailing various types of primary and secondary batteries, their chemical reactions, and applications in different devices. It highlights the importance of battery technology in addressing energy concerns, environmental impacts, and the future of electric vehicles. Additionally, it covers the performance factors, testing methods, and potential issues affecting battery life, particularly focusing on lead-acid and lithium batteries.