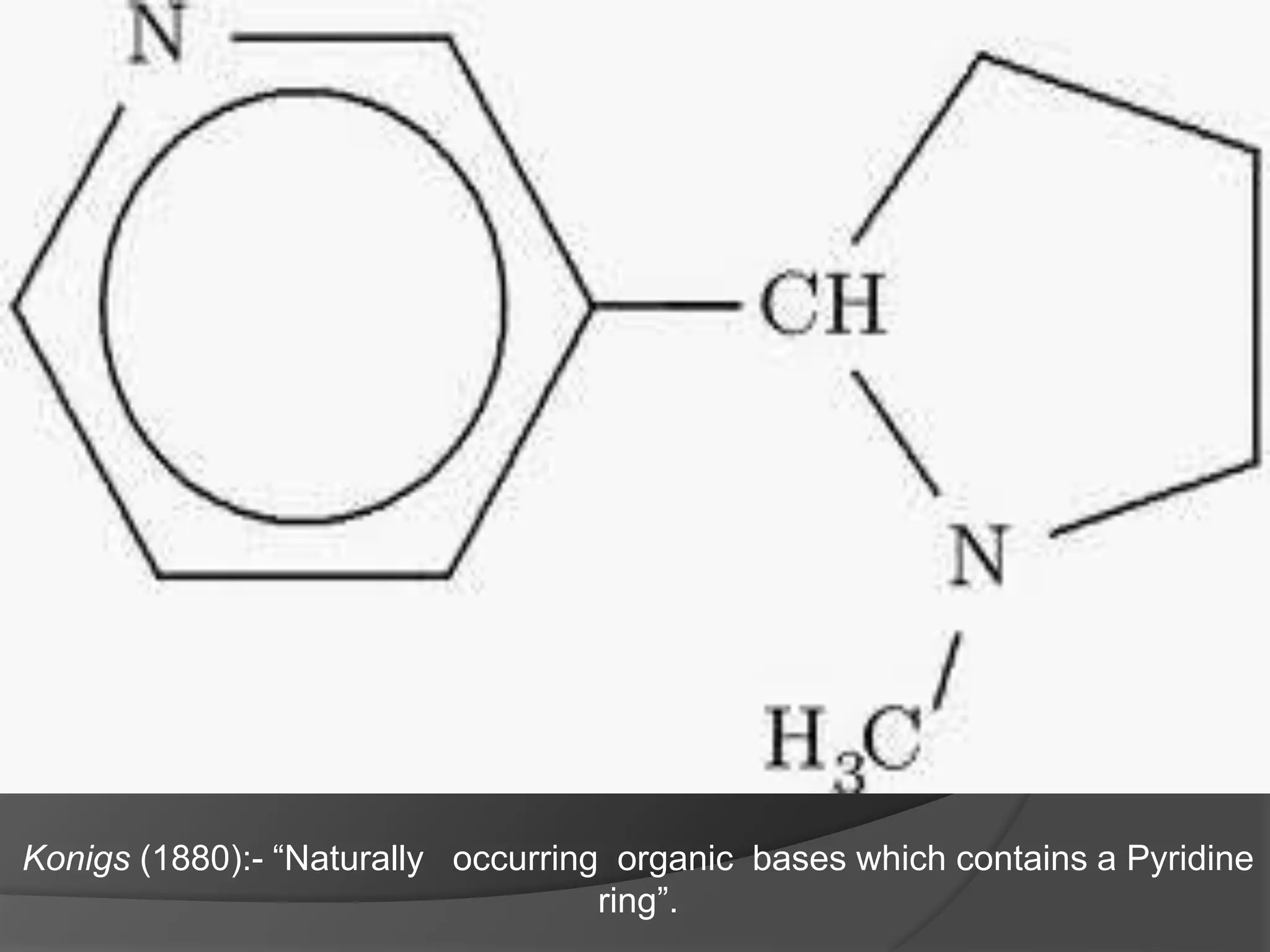
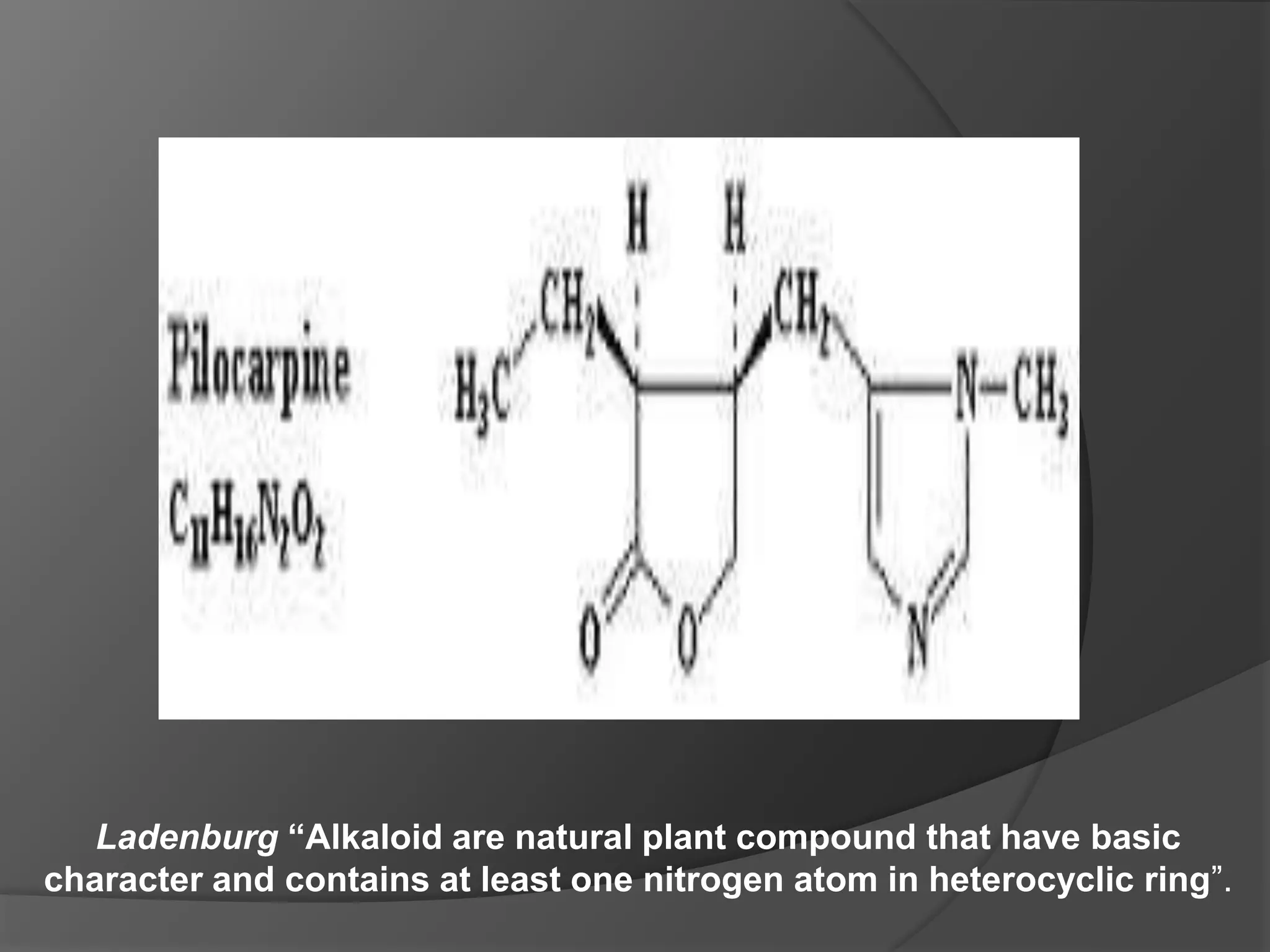
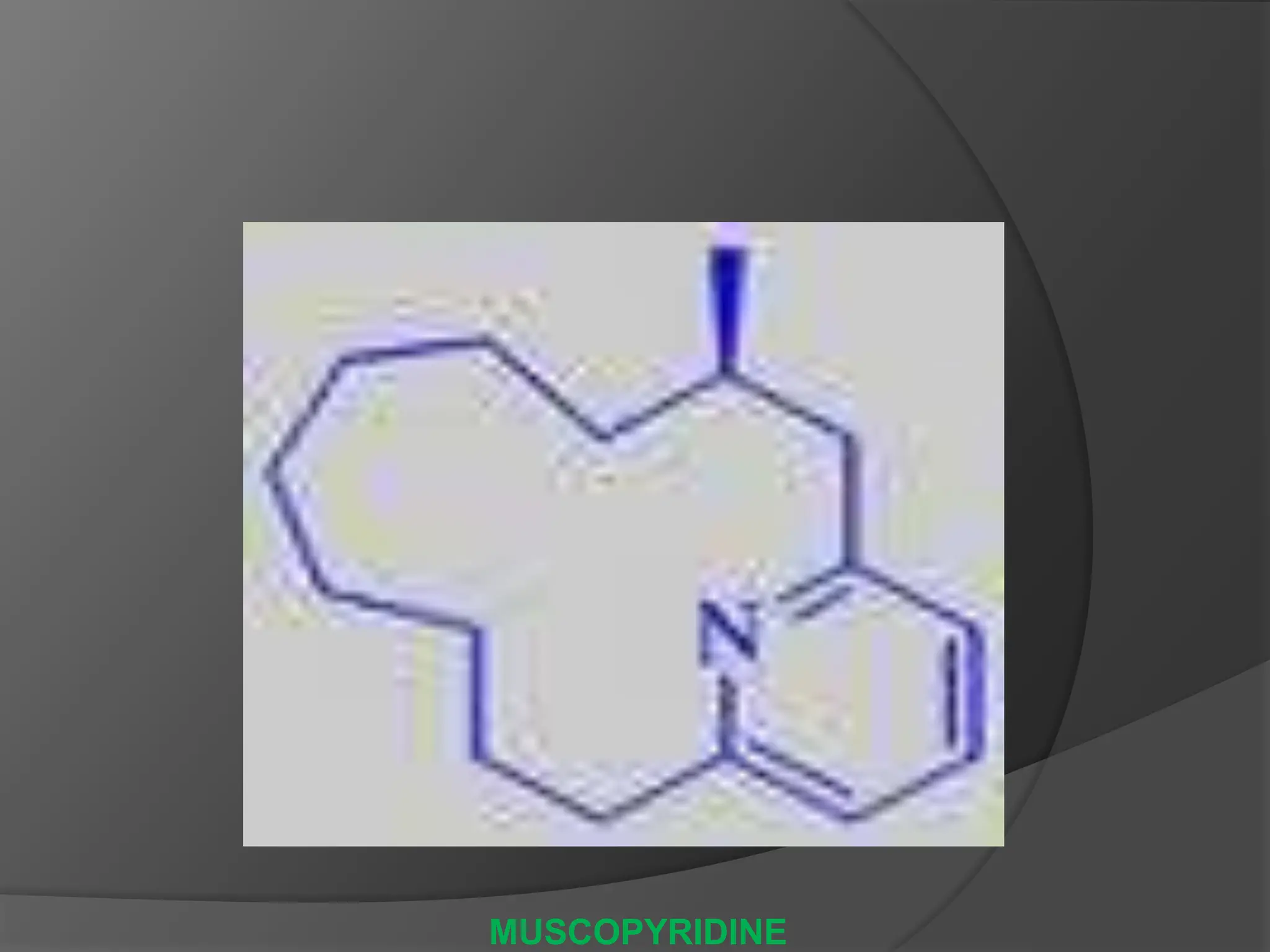
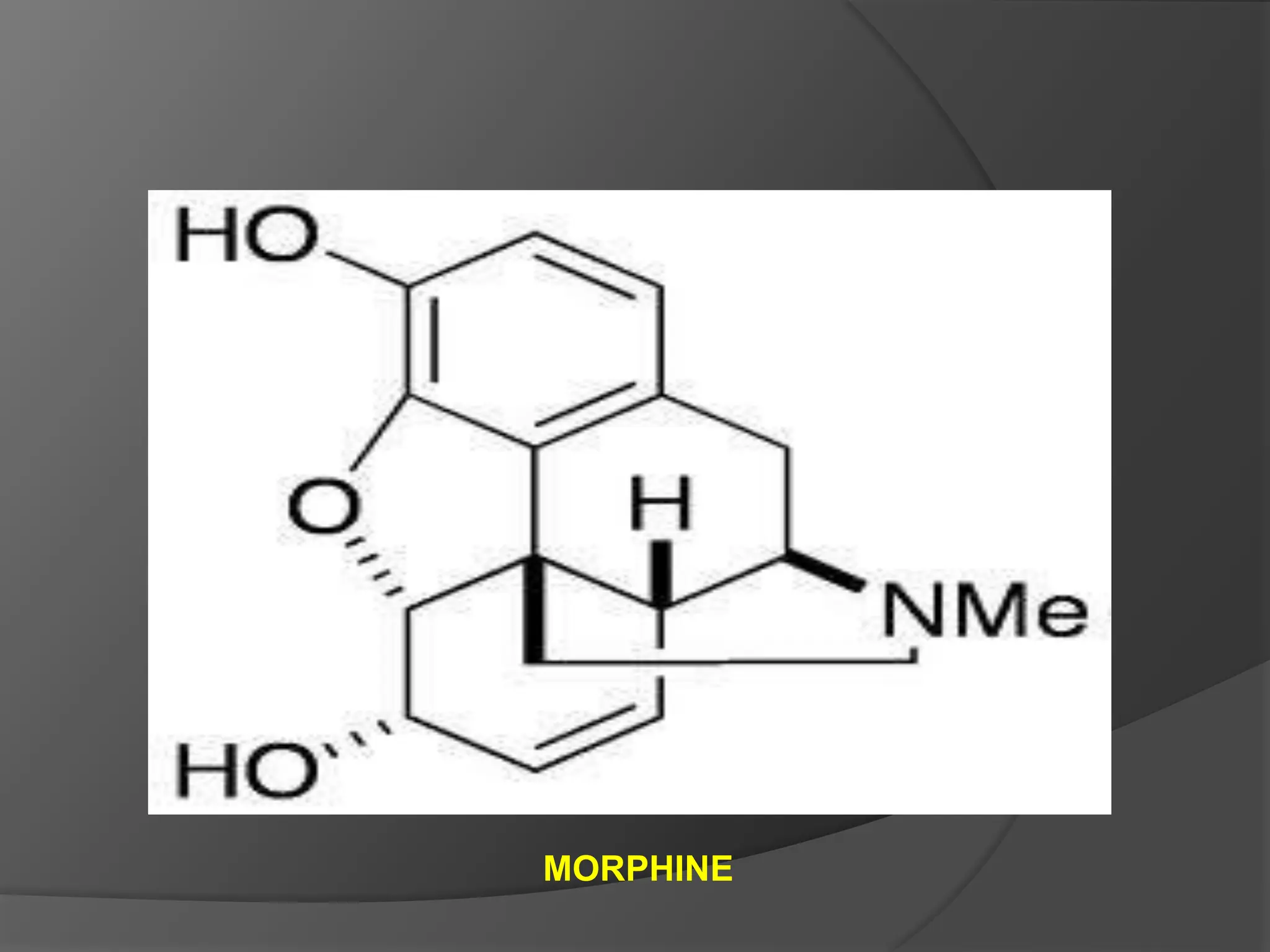
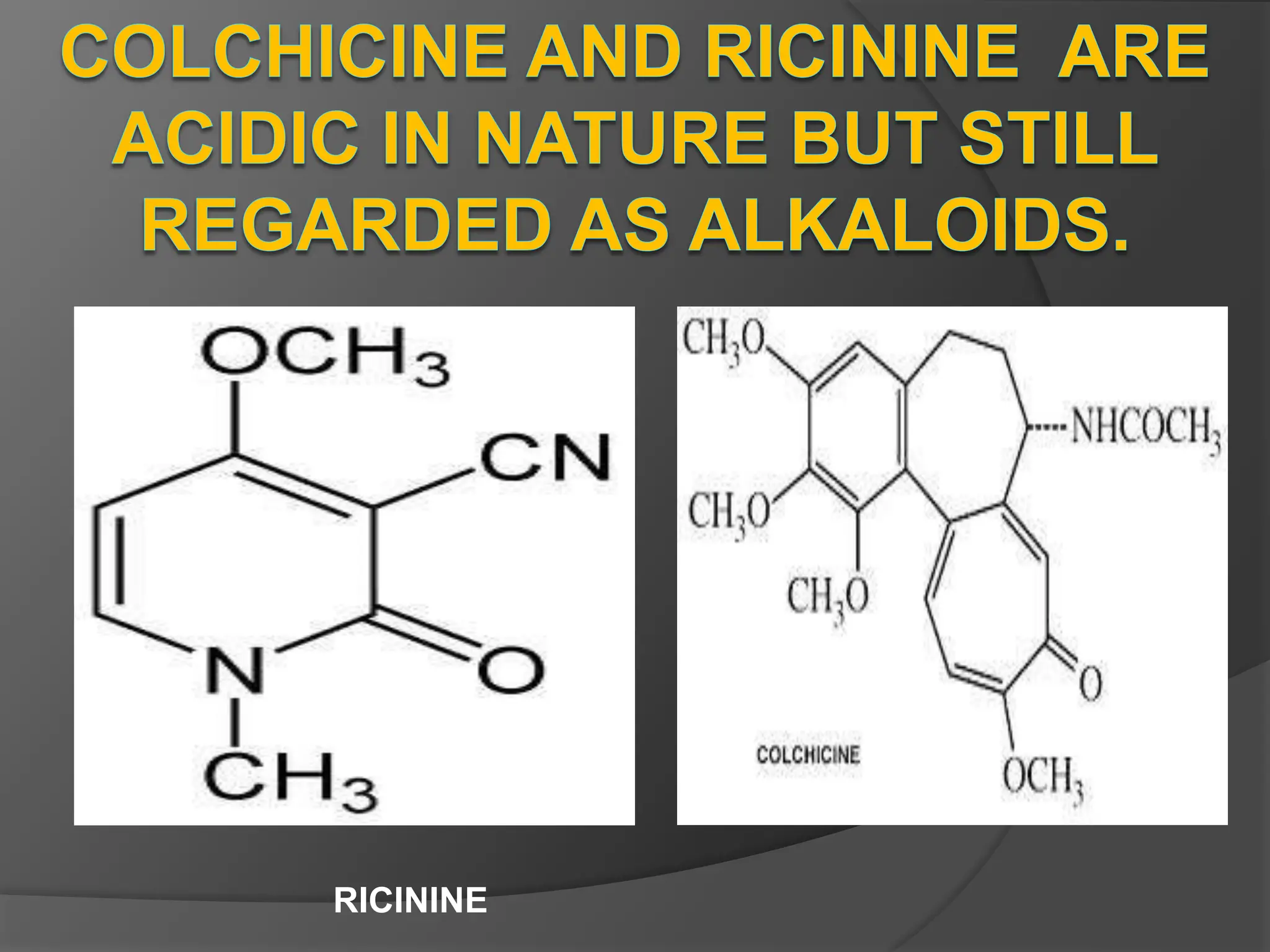
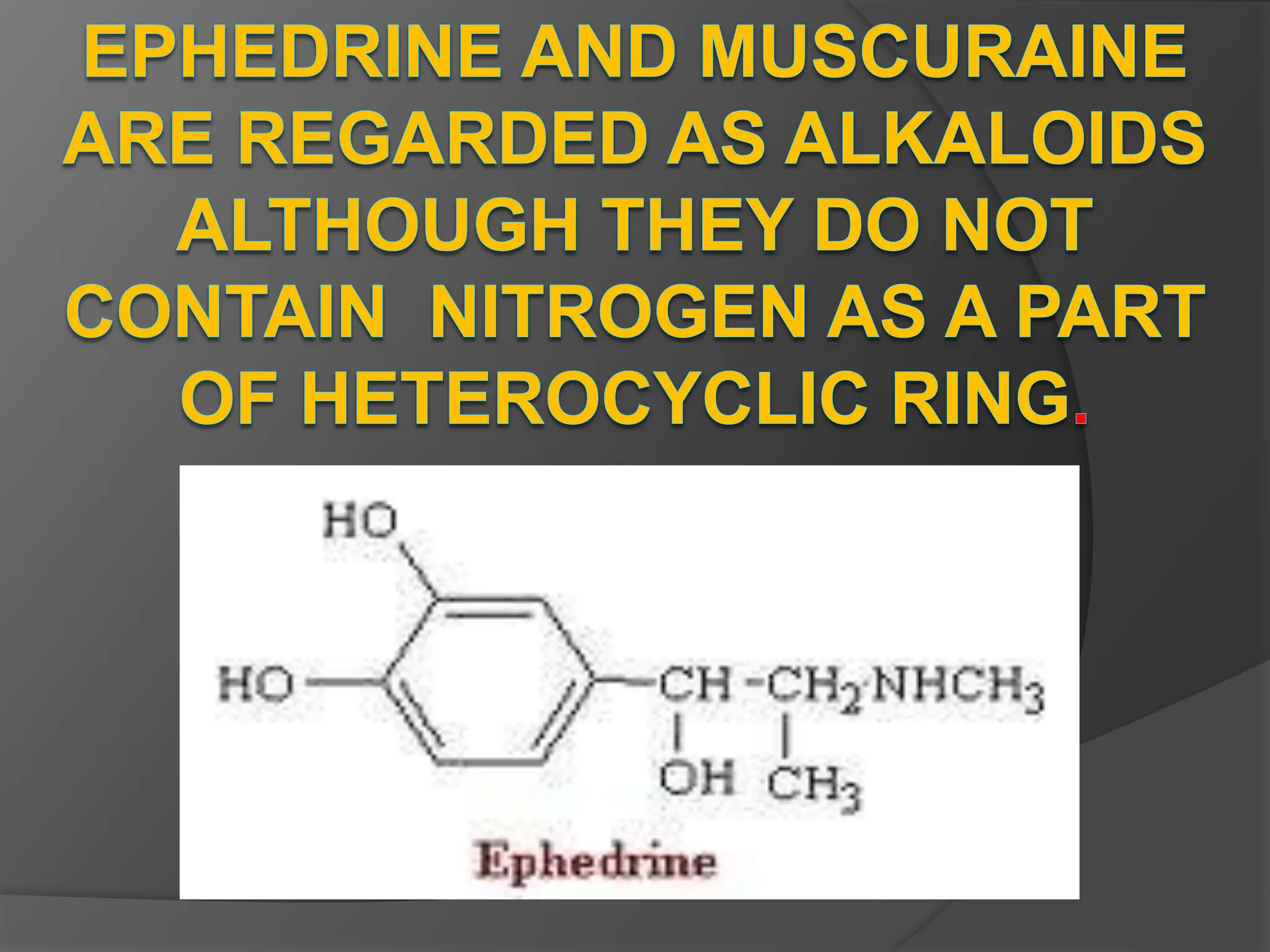
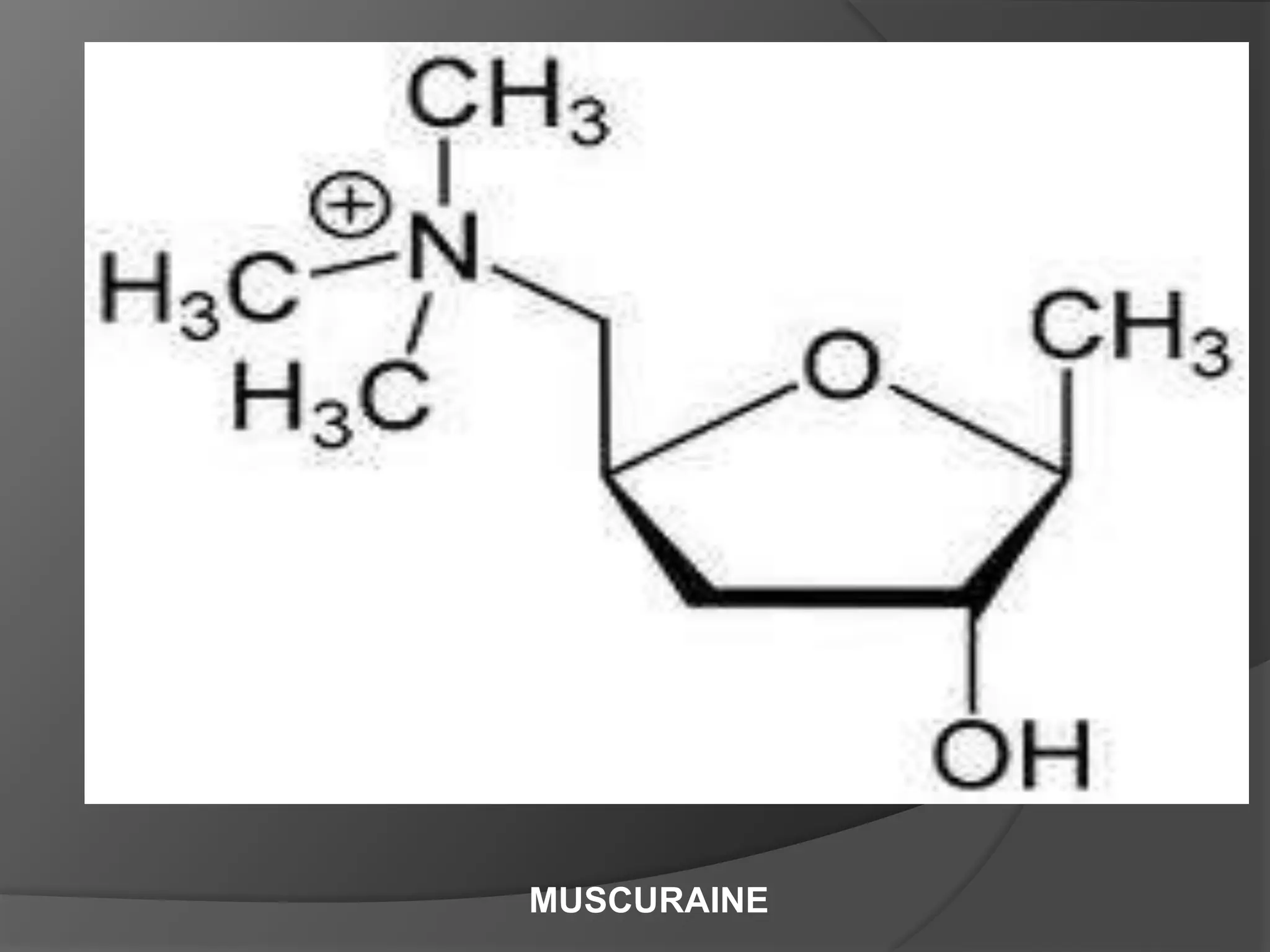
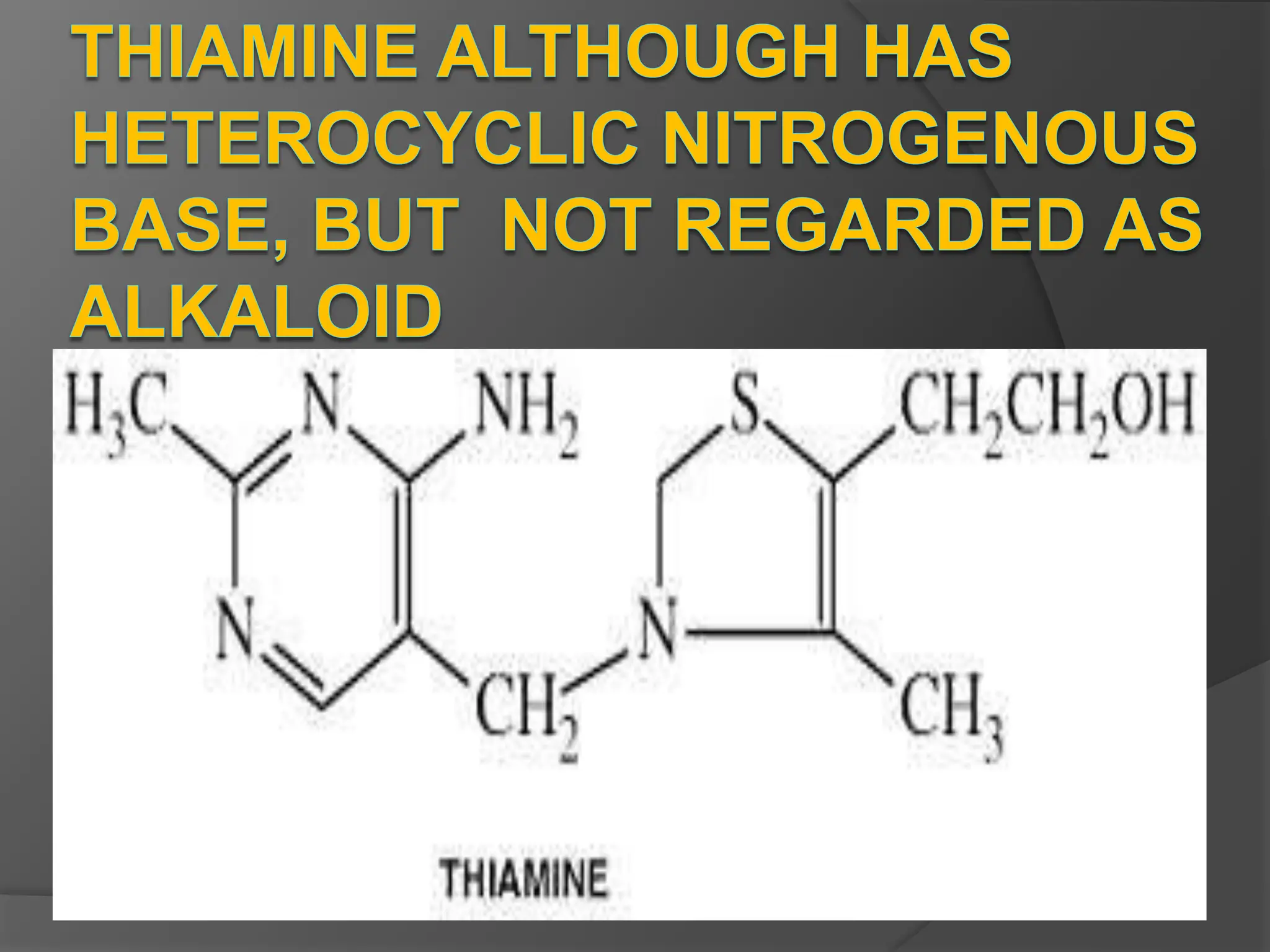
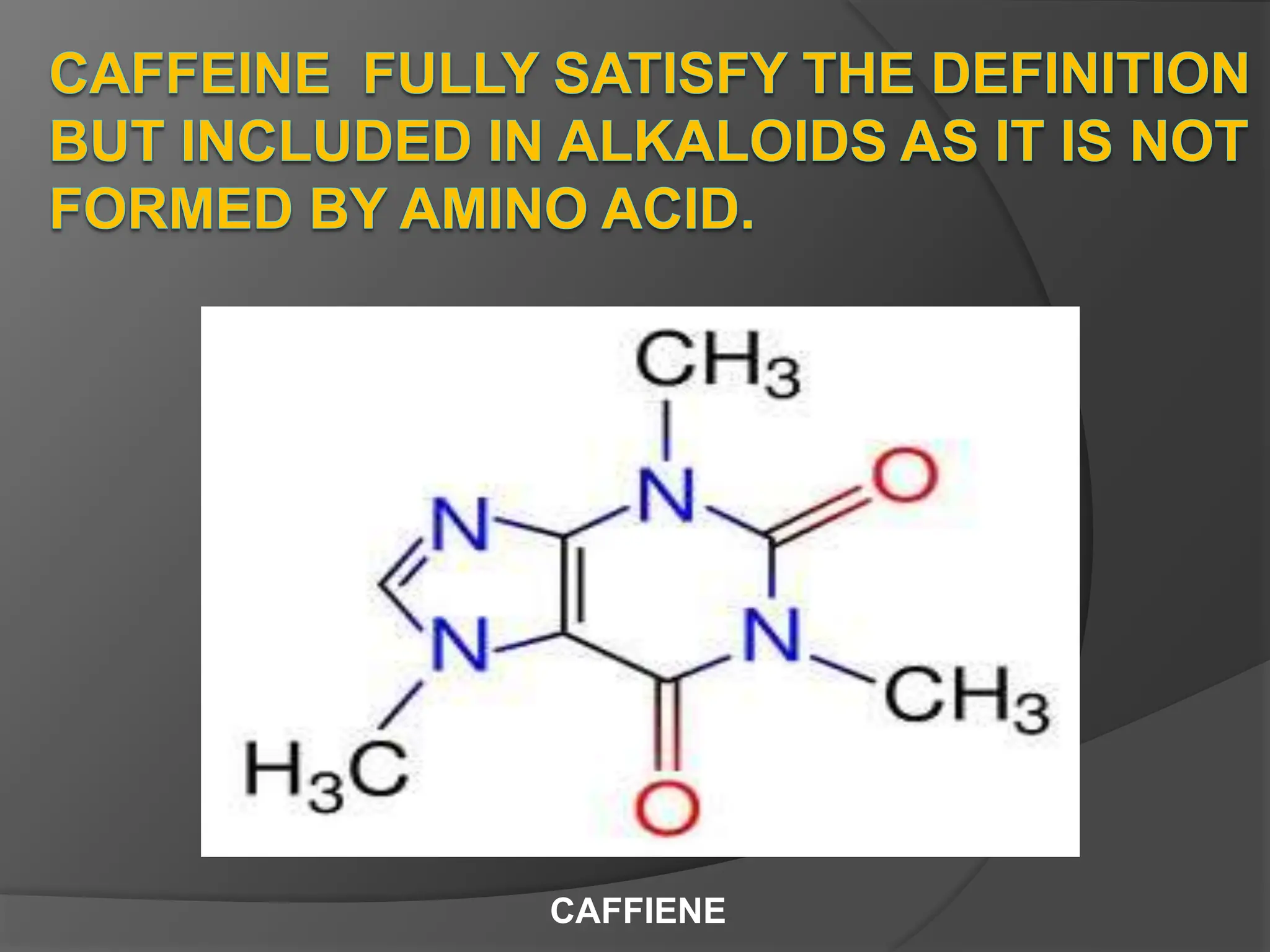
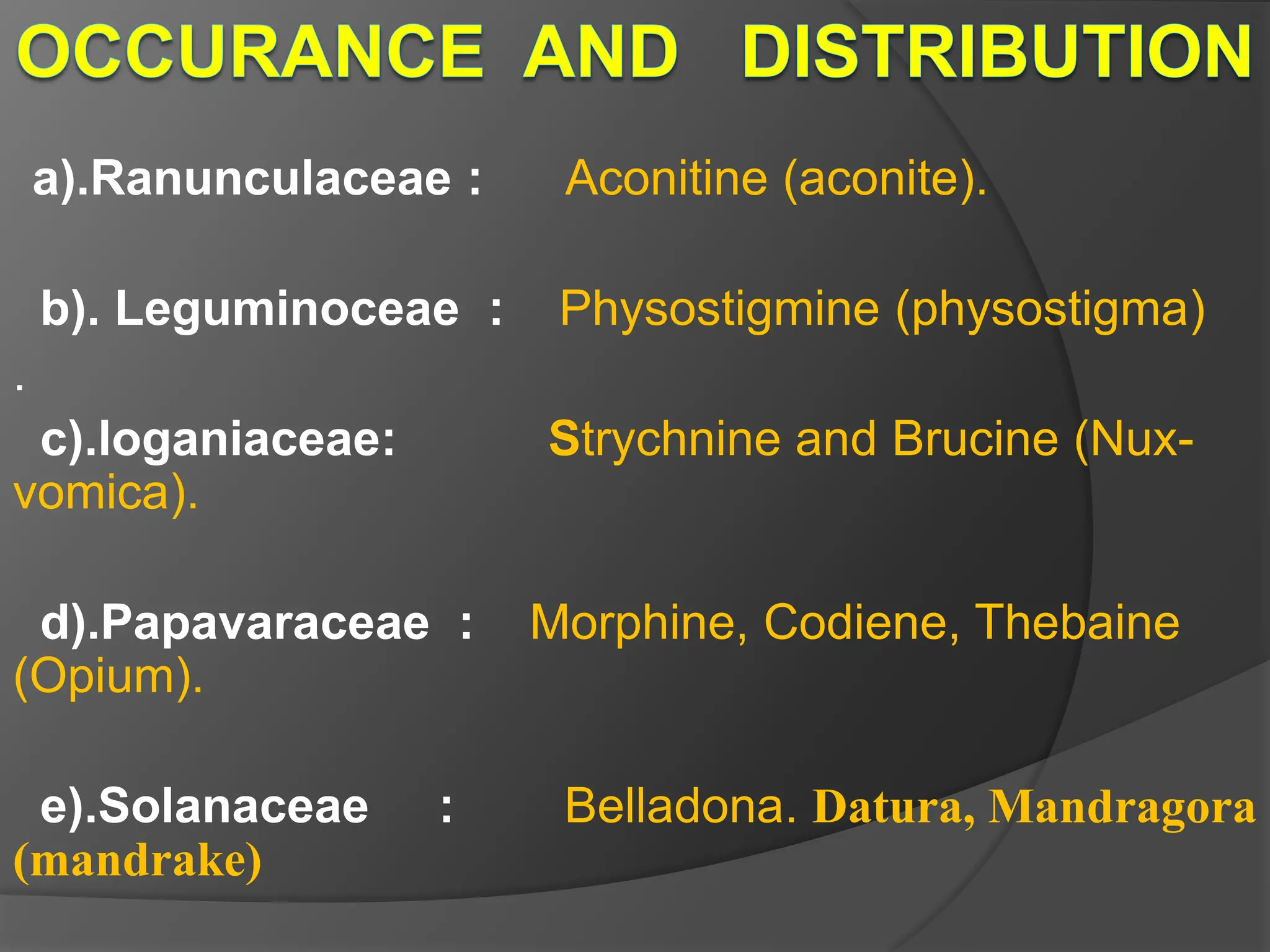
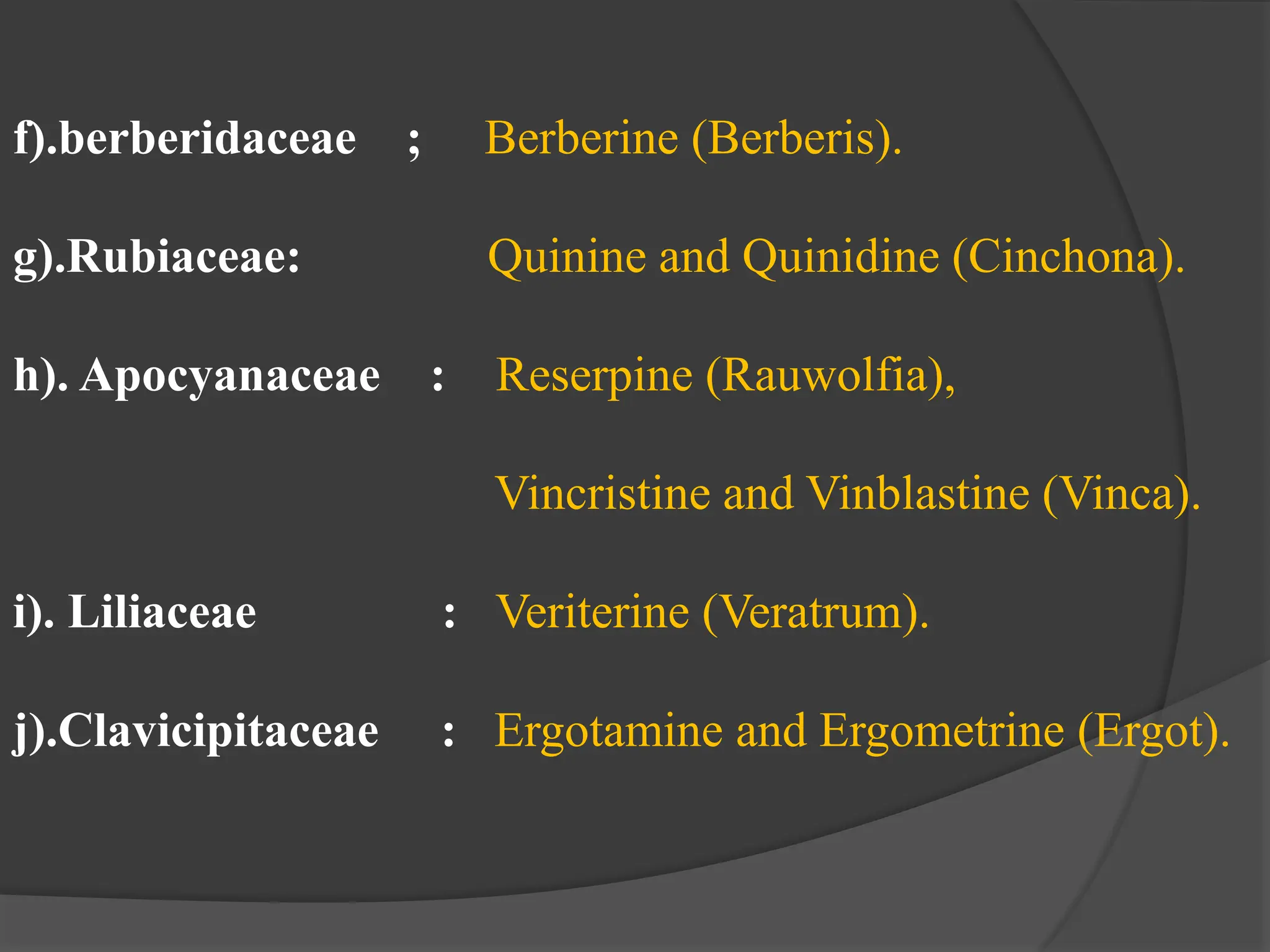
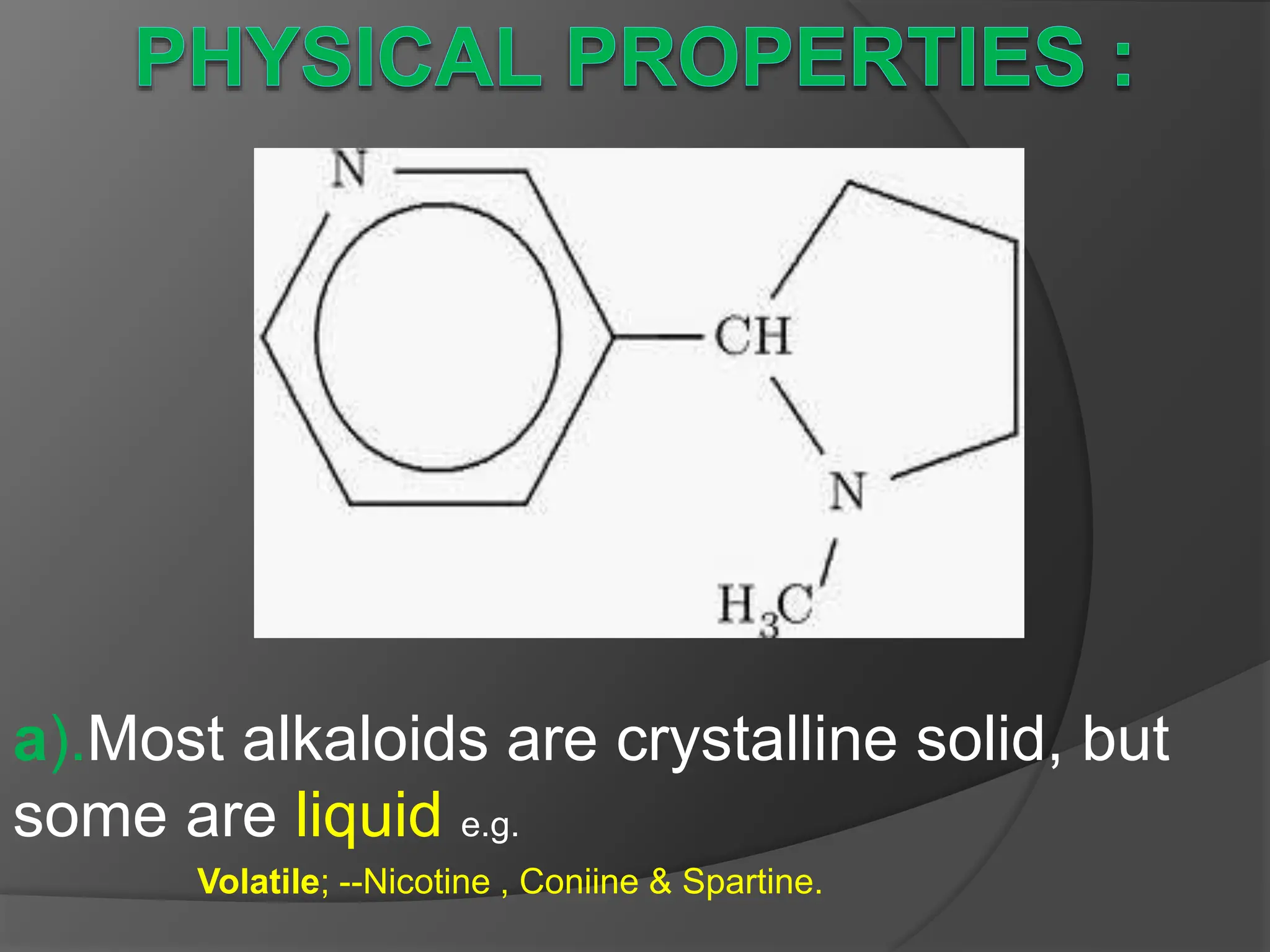
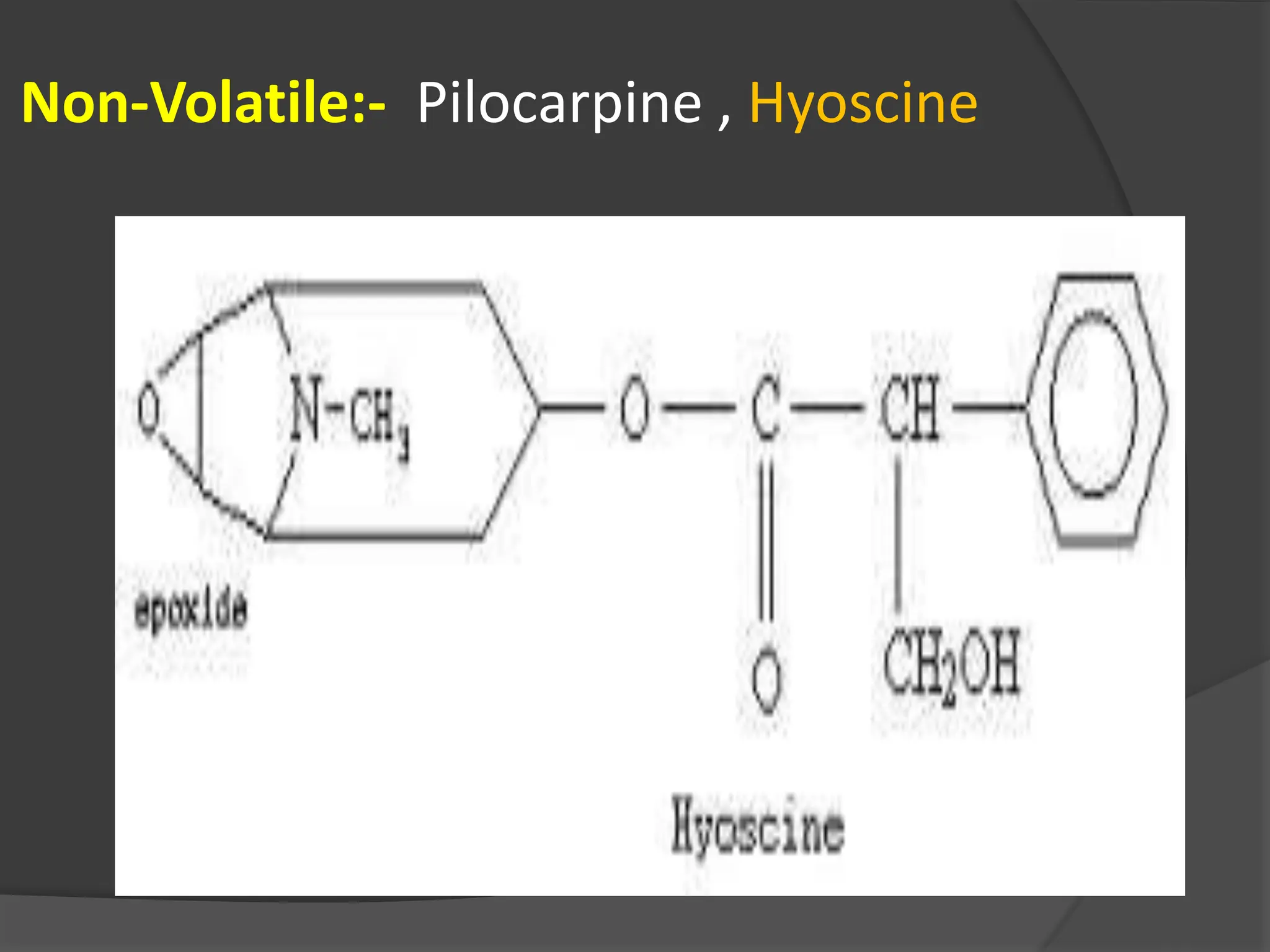
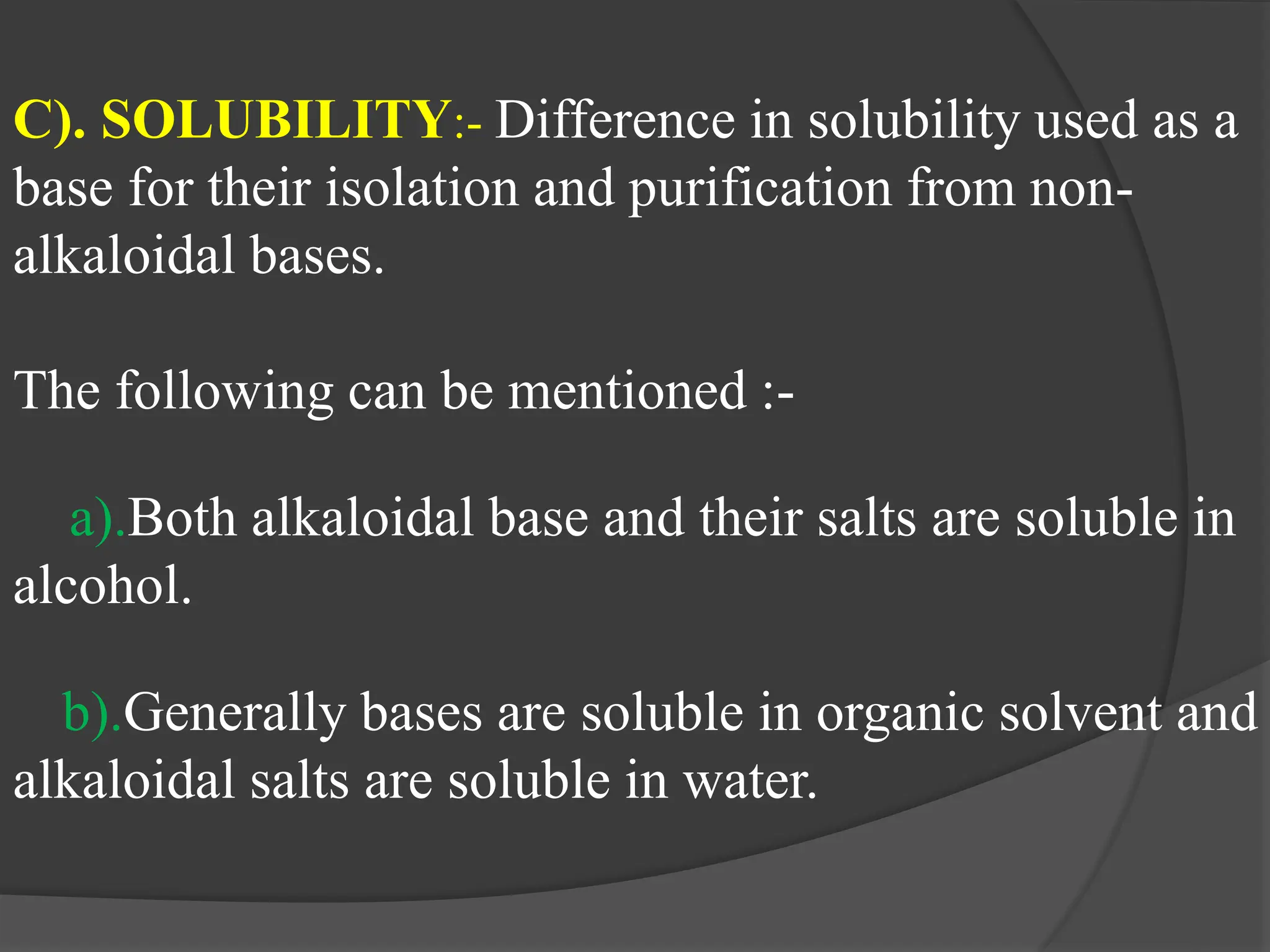
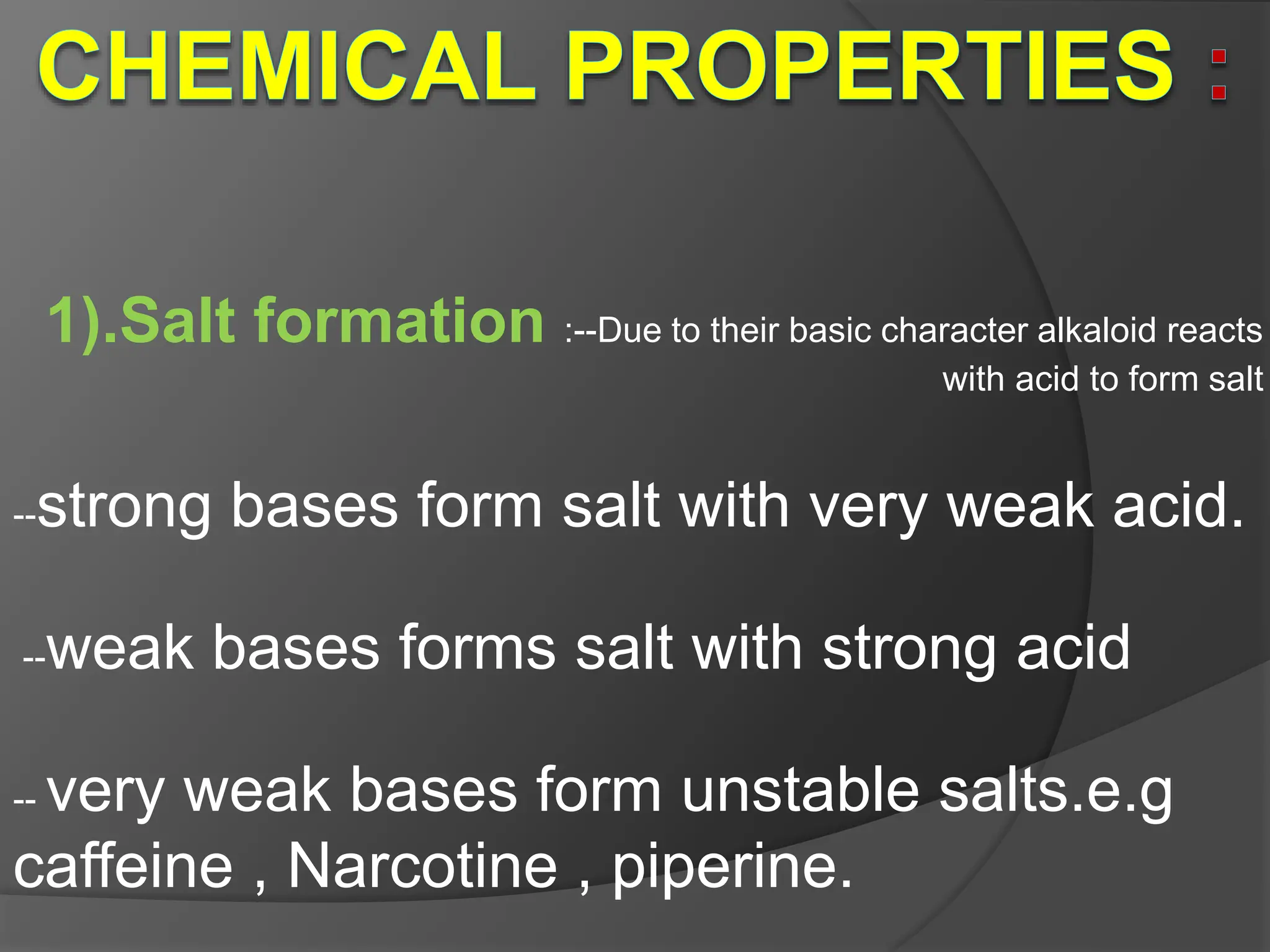
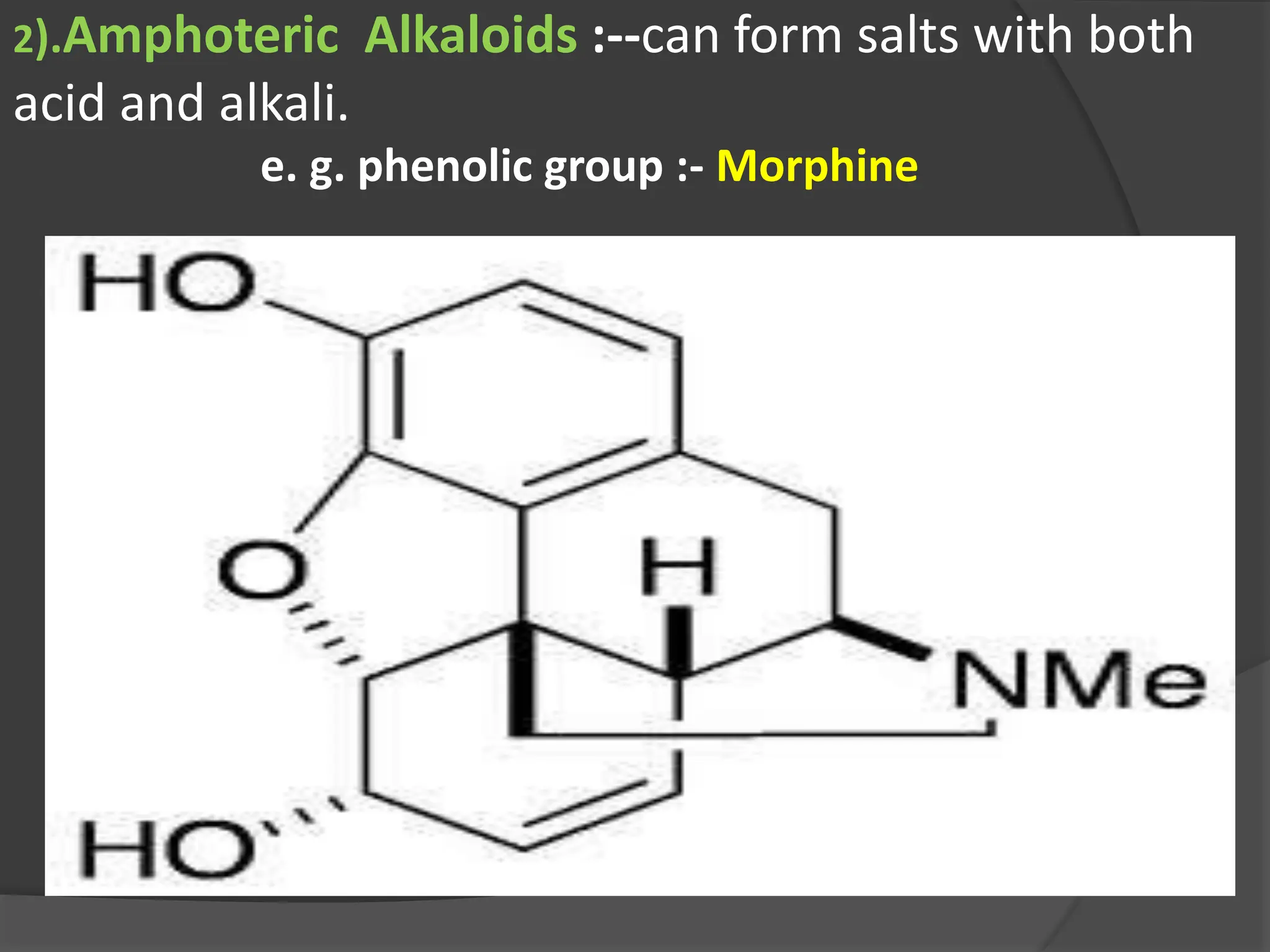
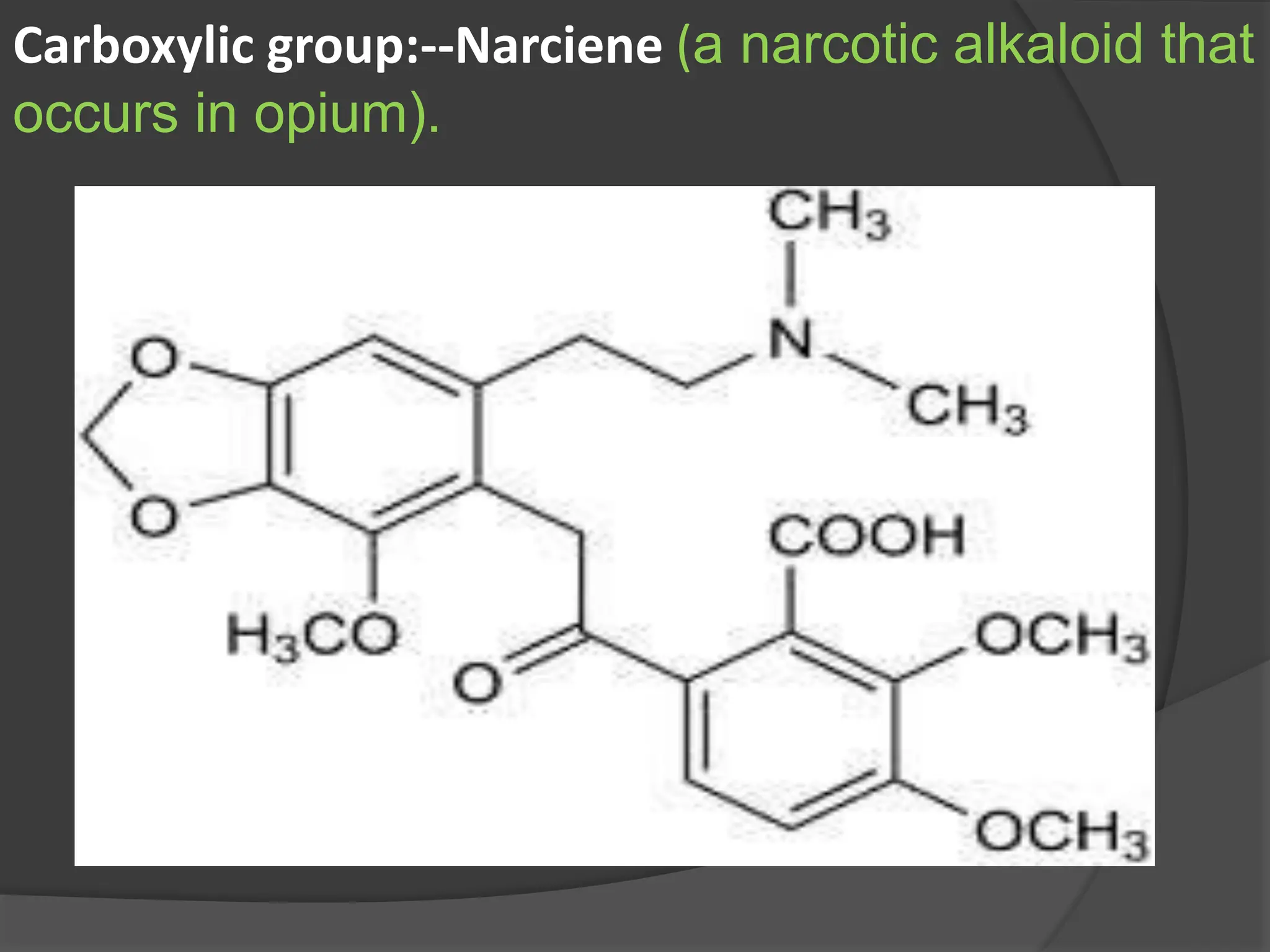
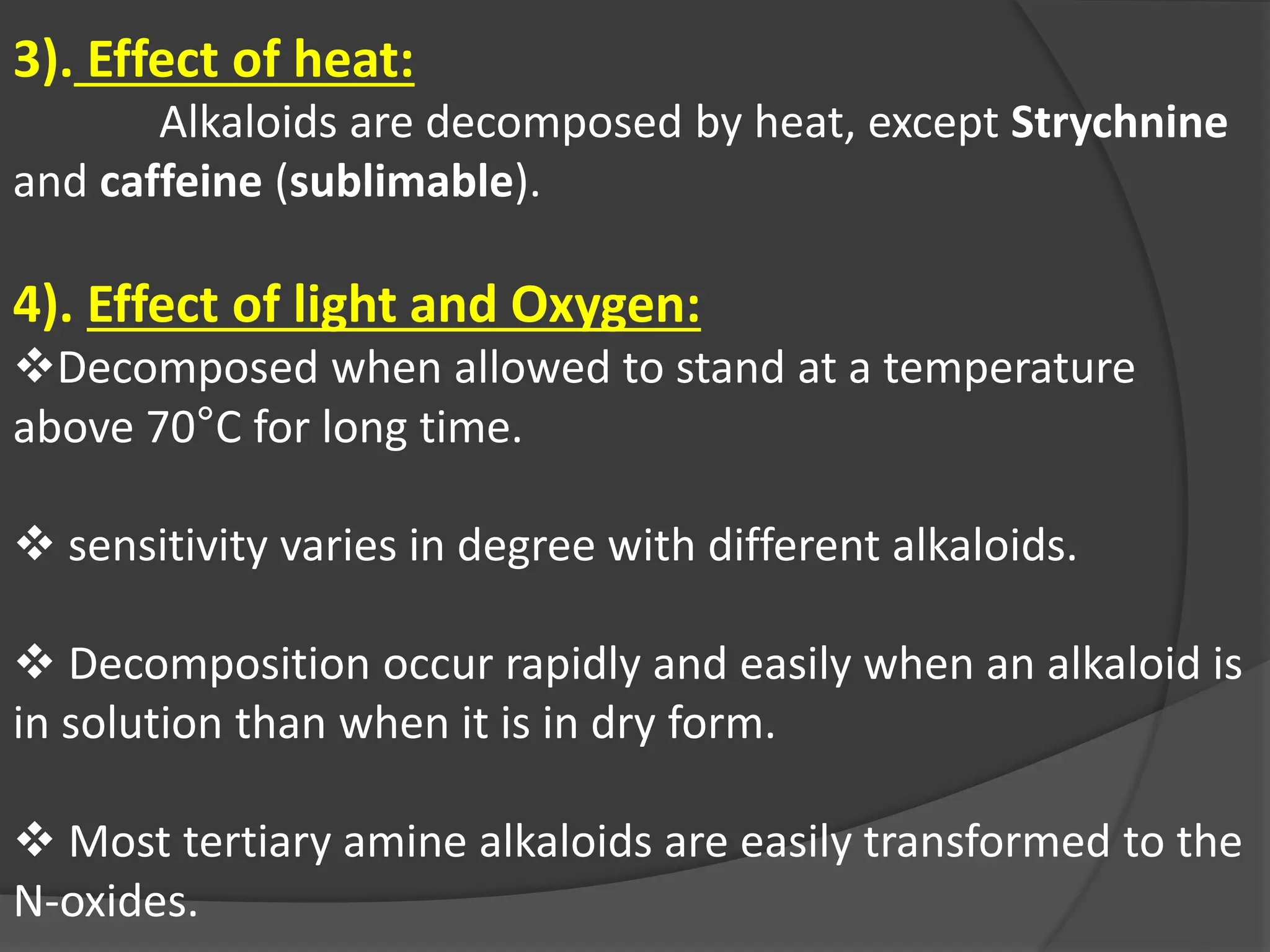
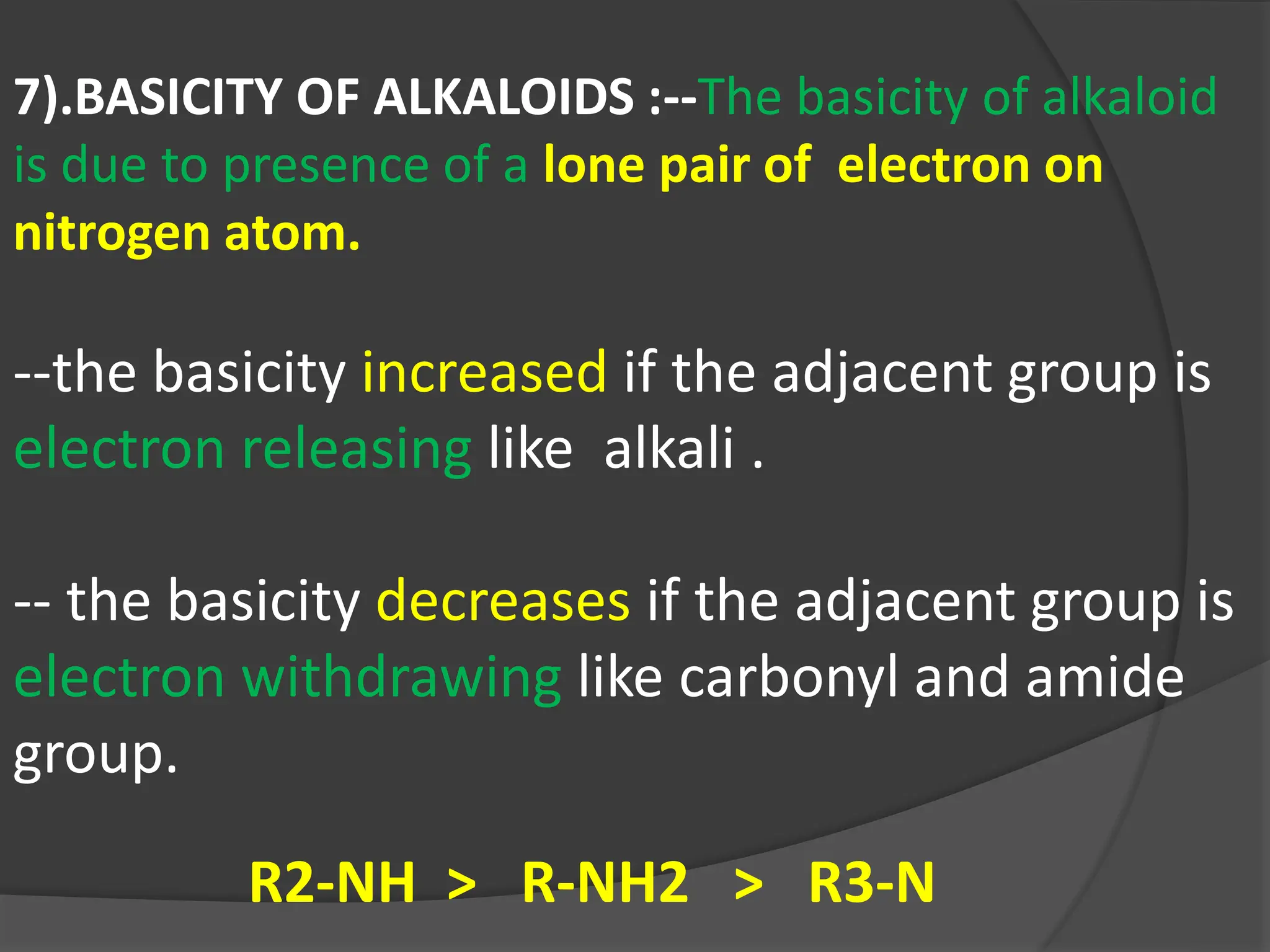
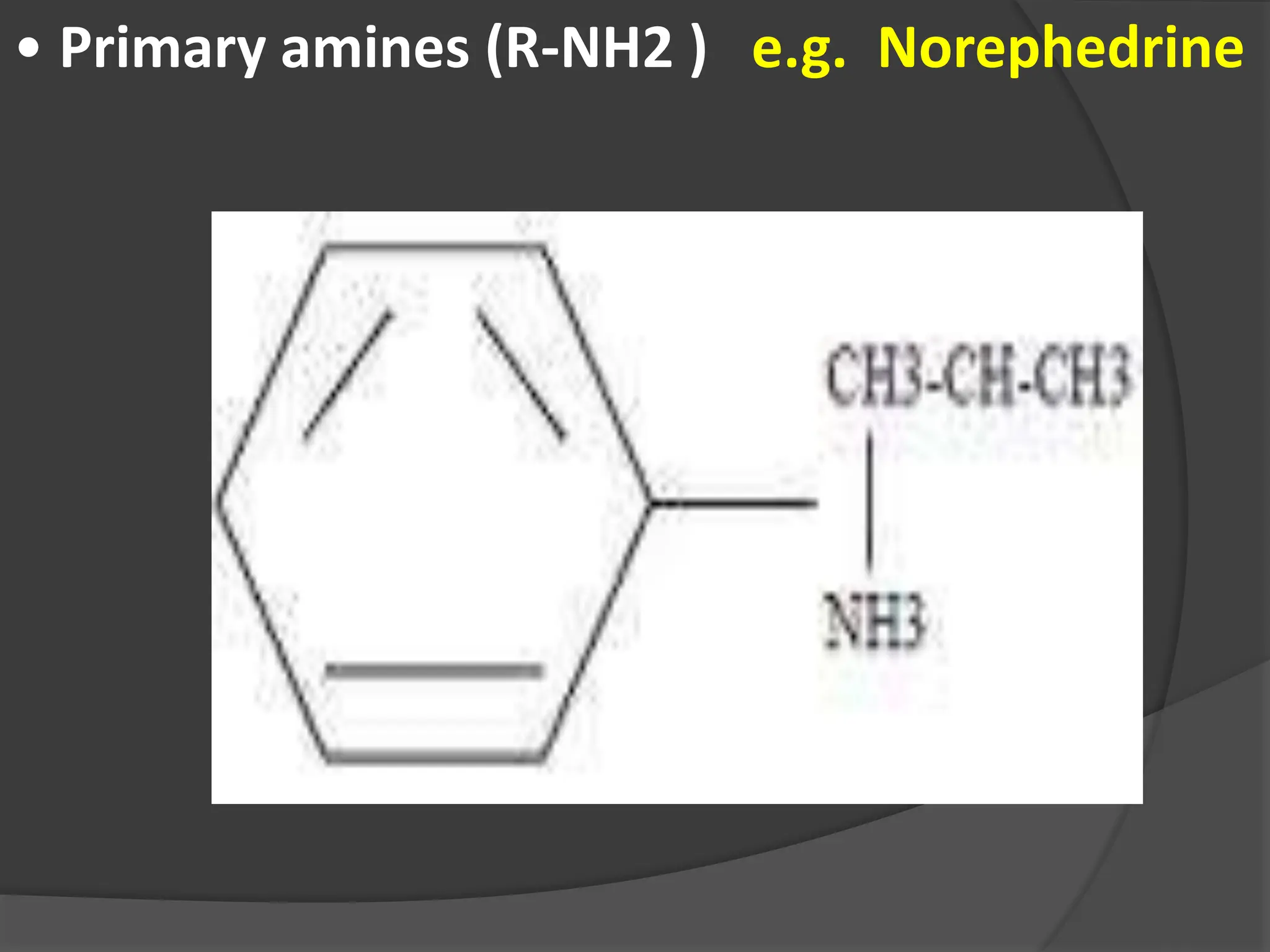
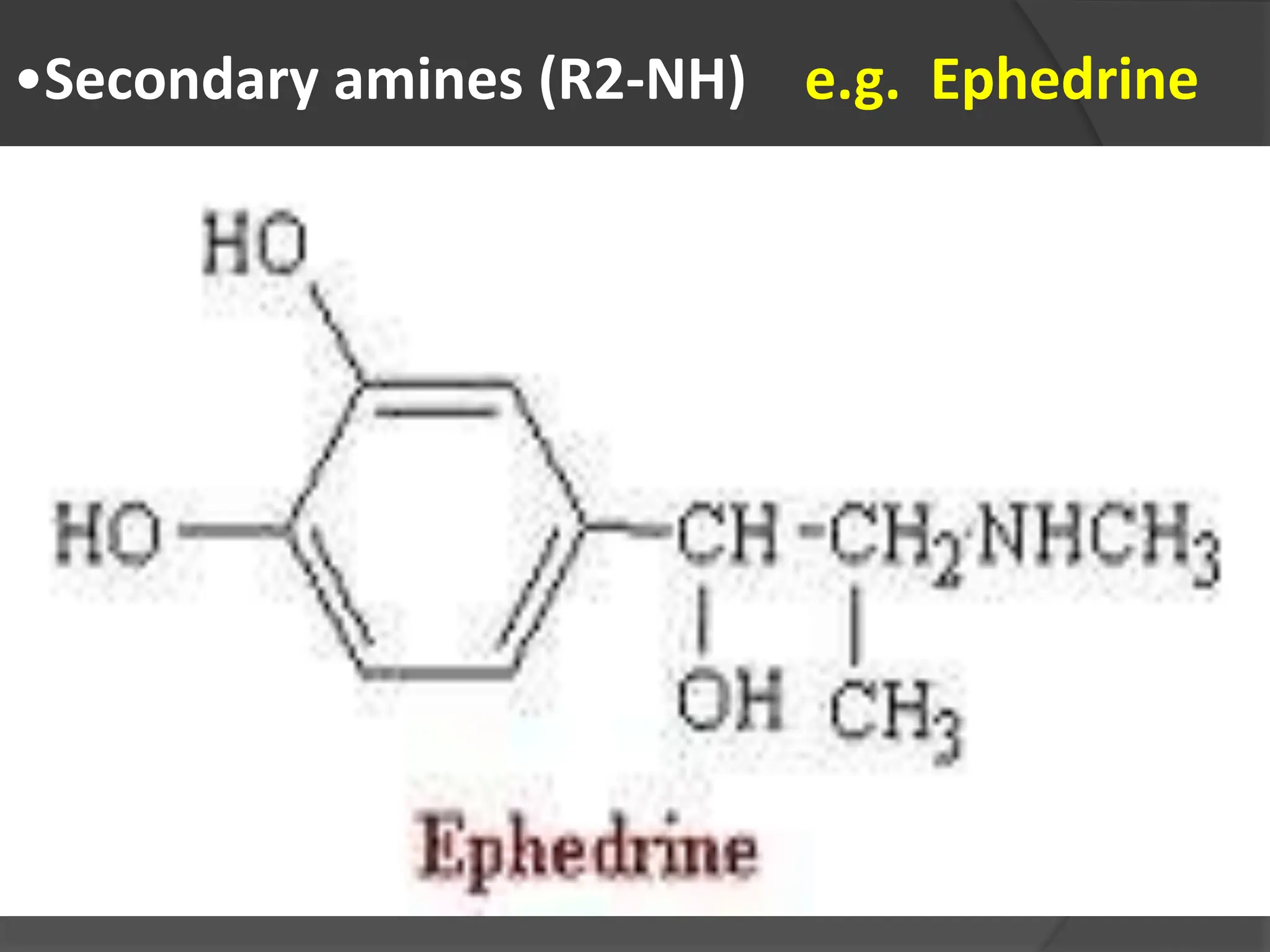
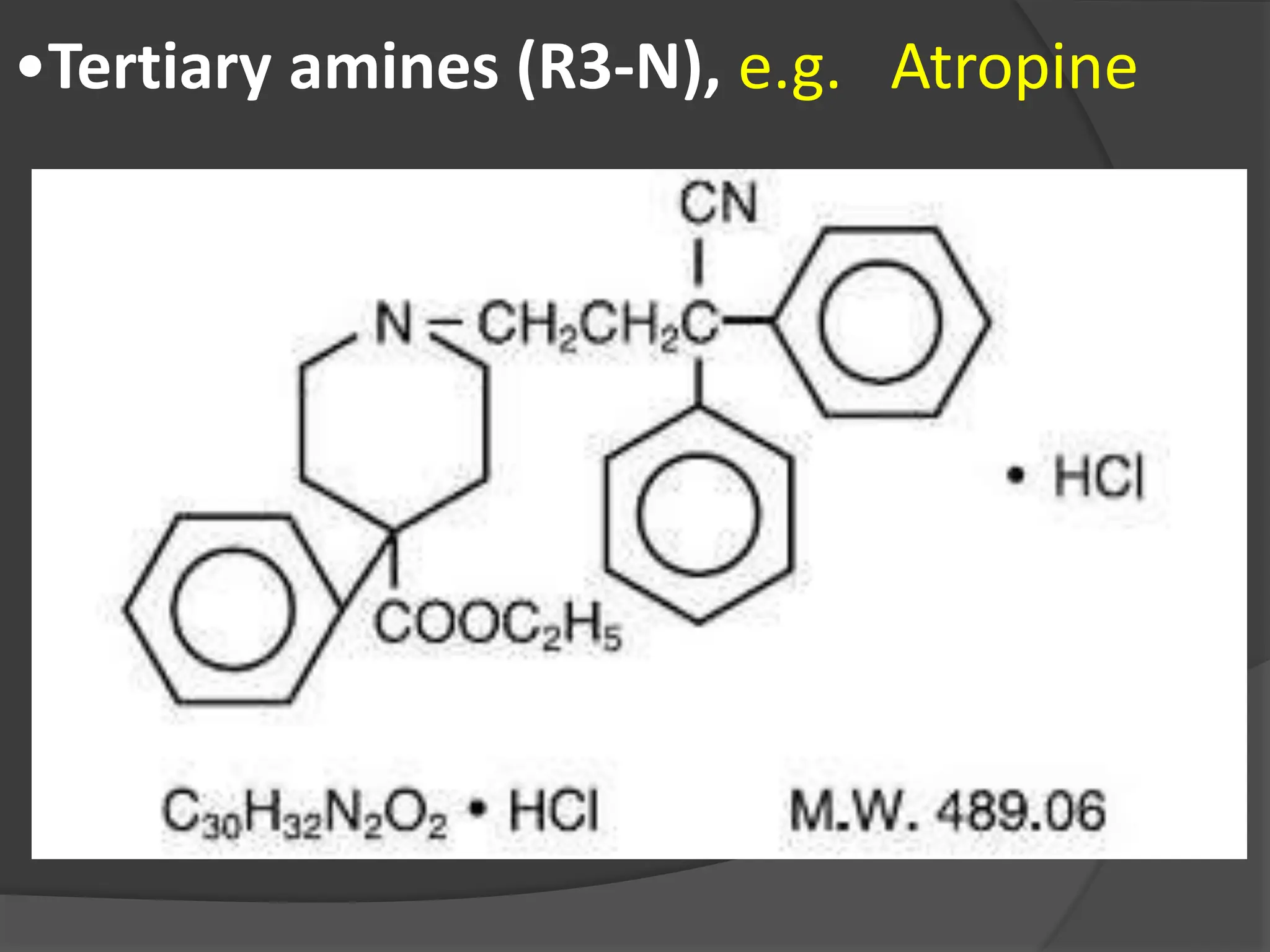
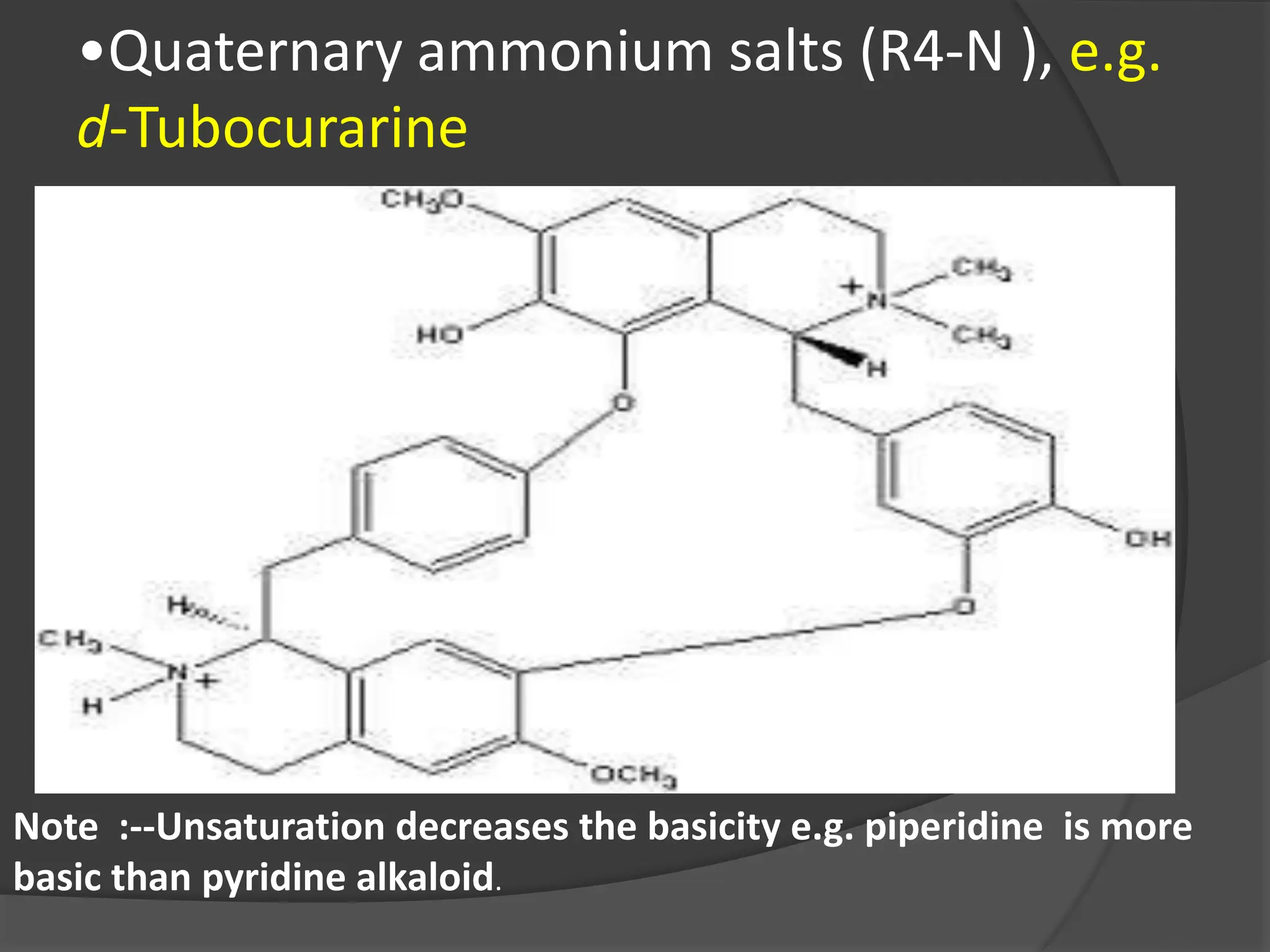
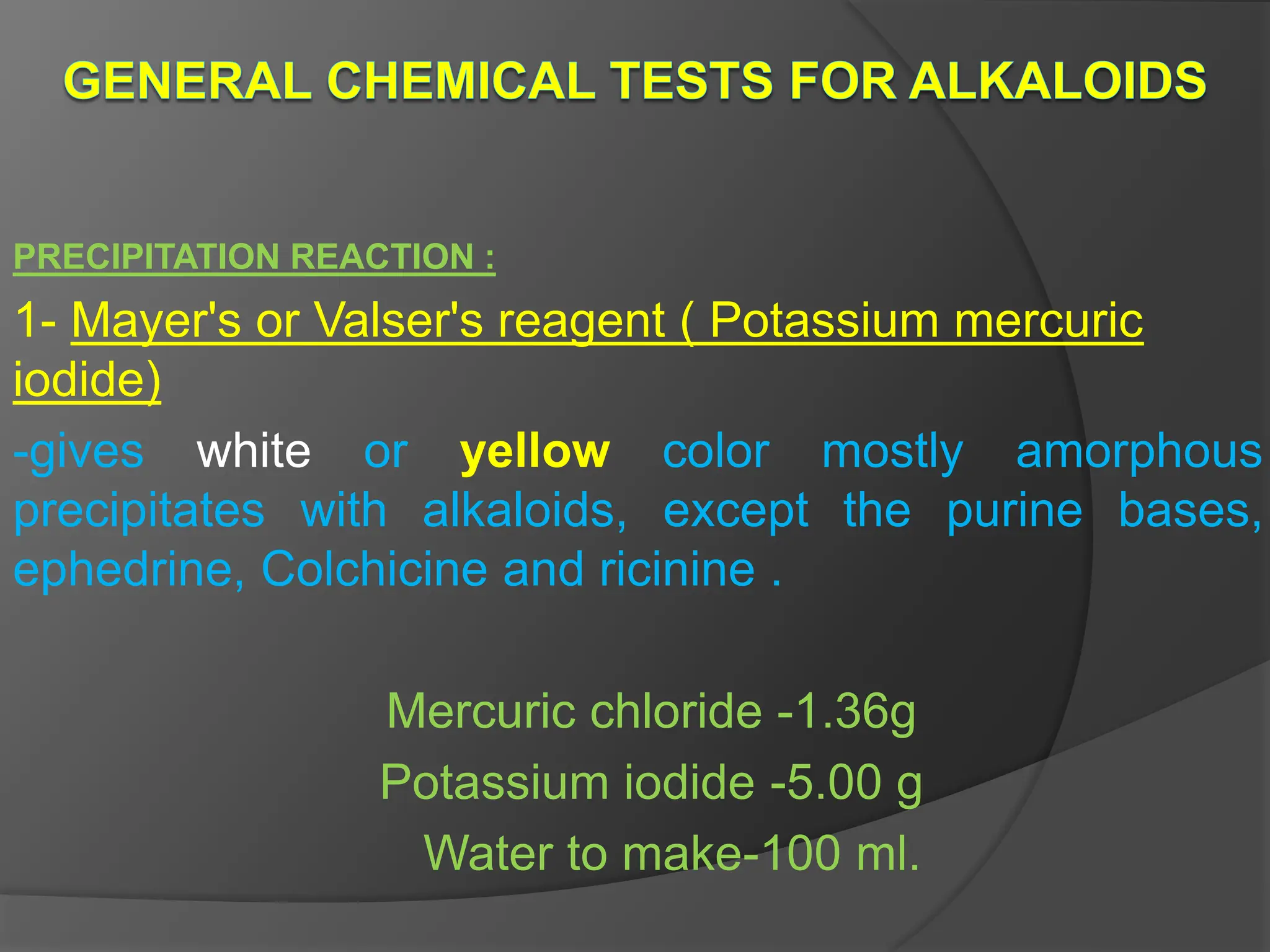
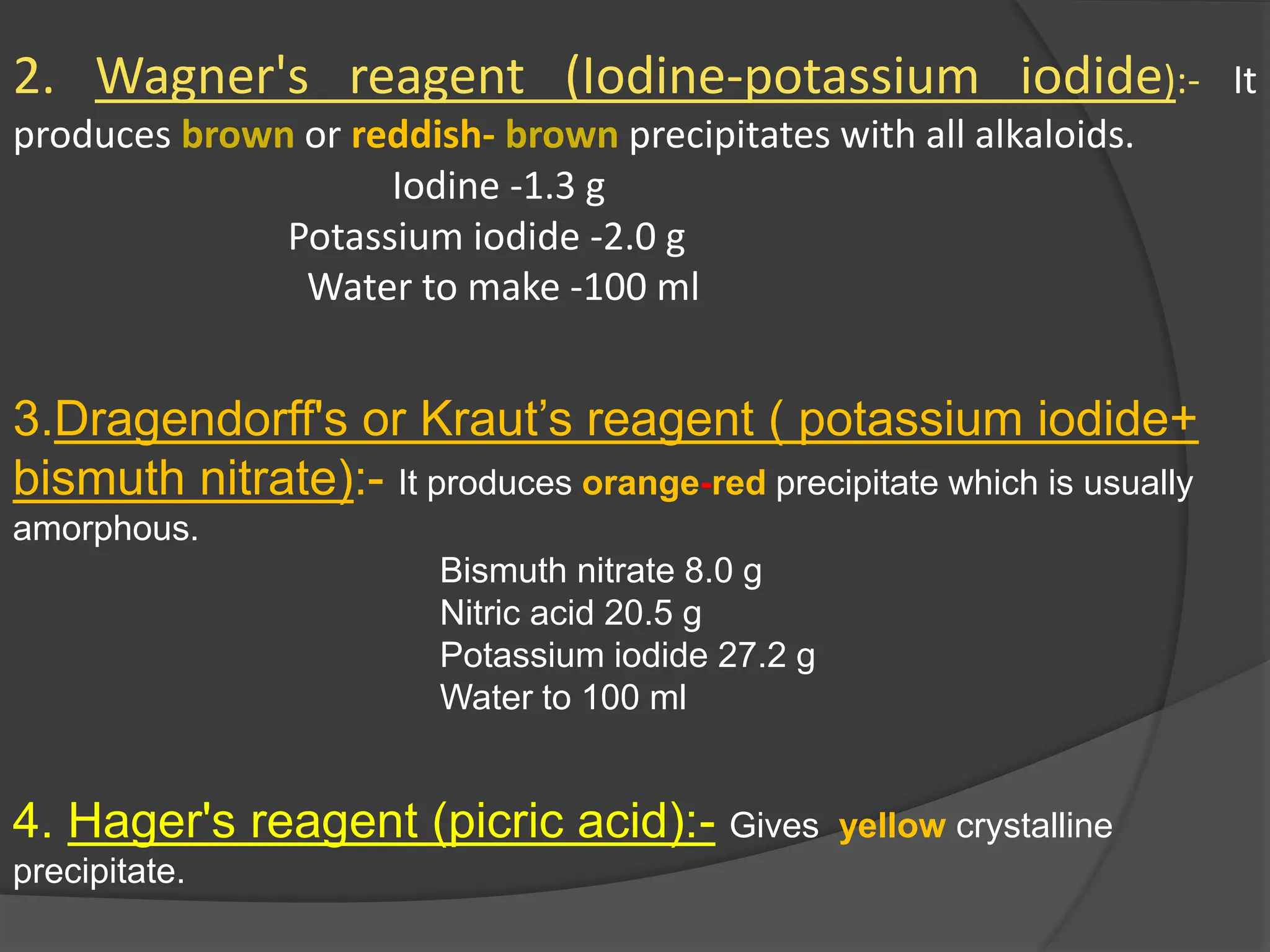
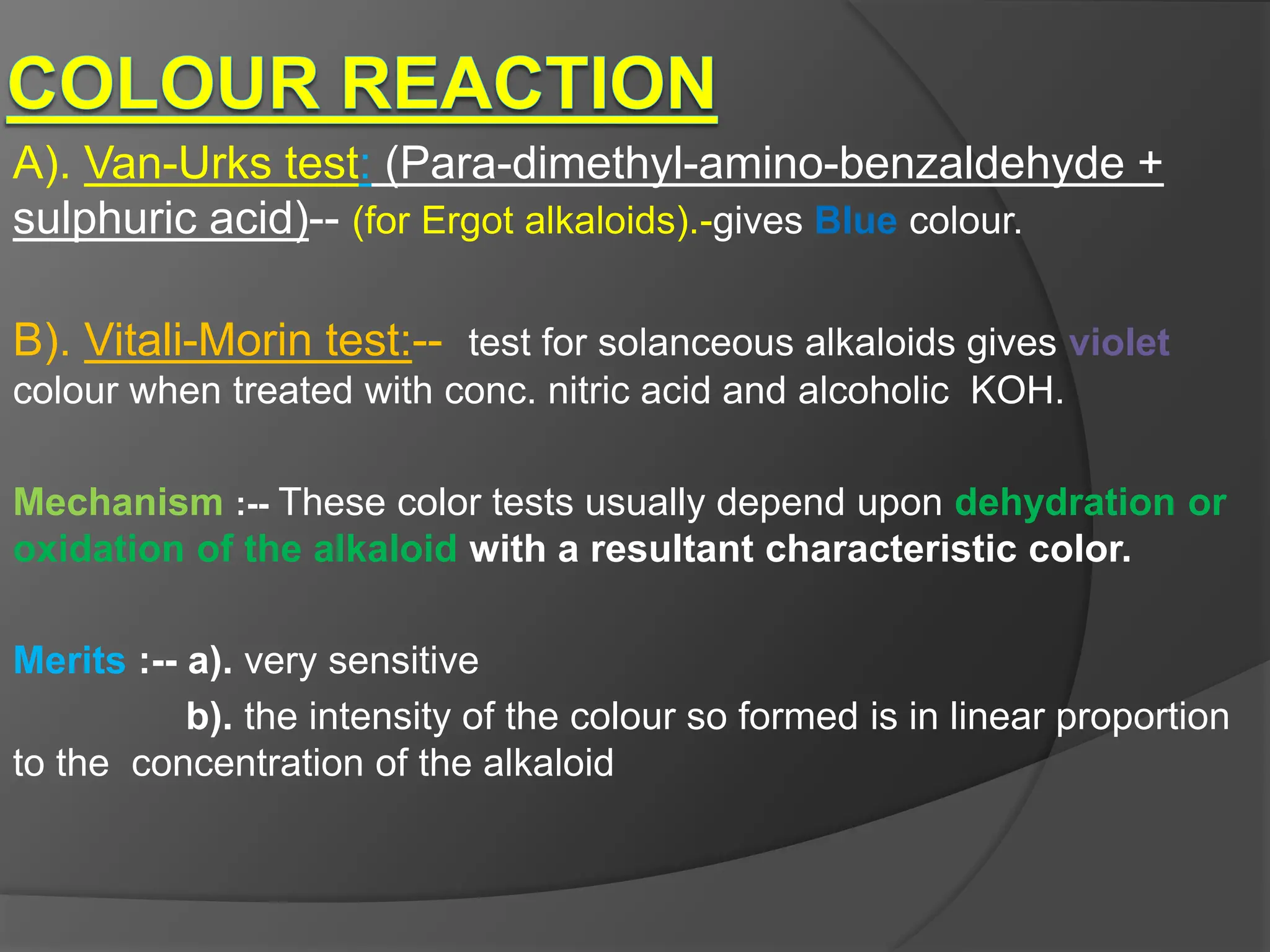
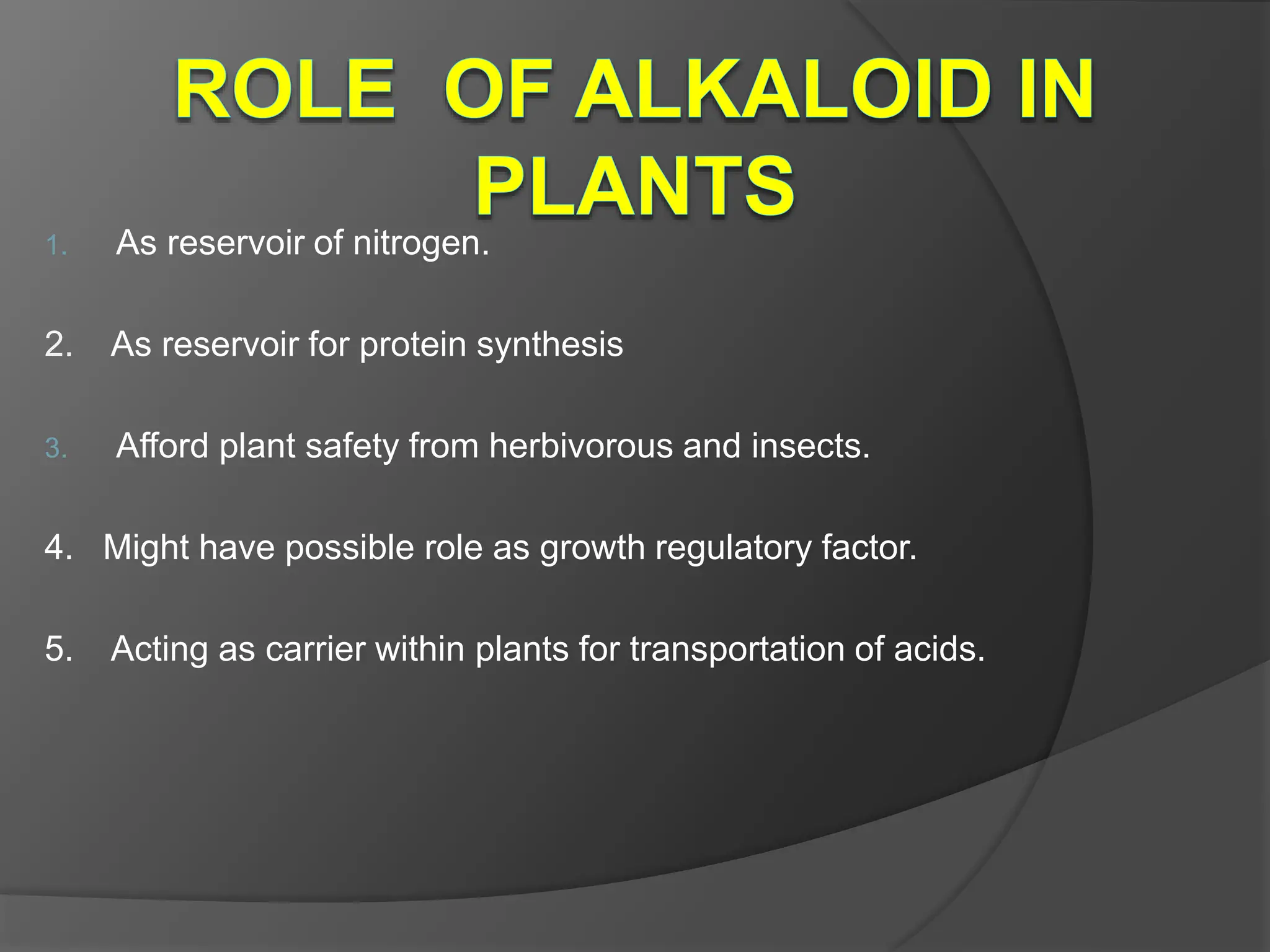
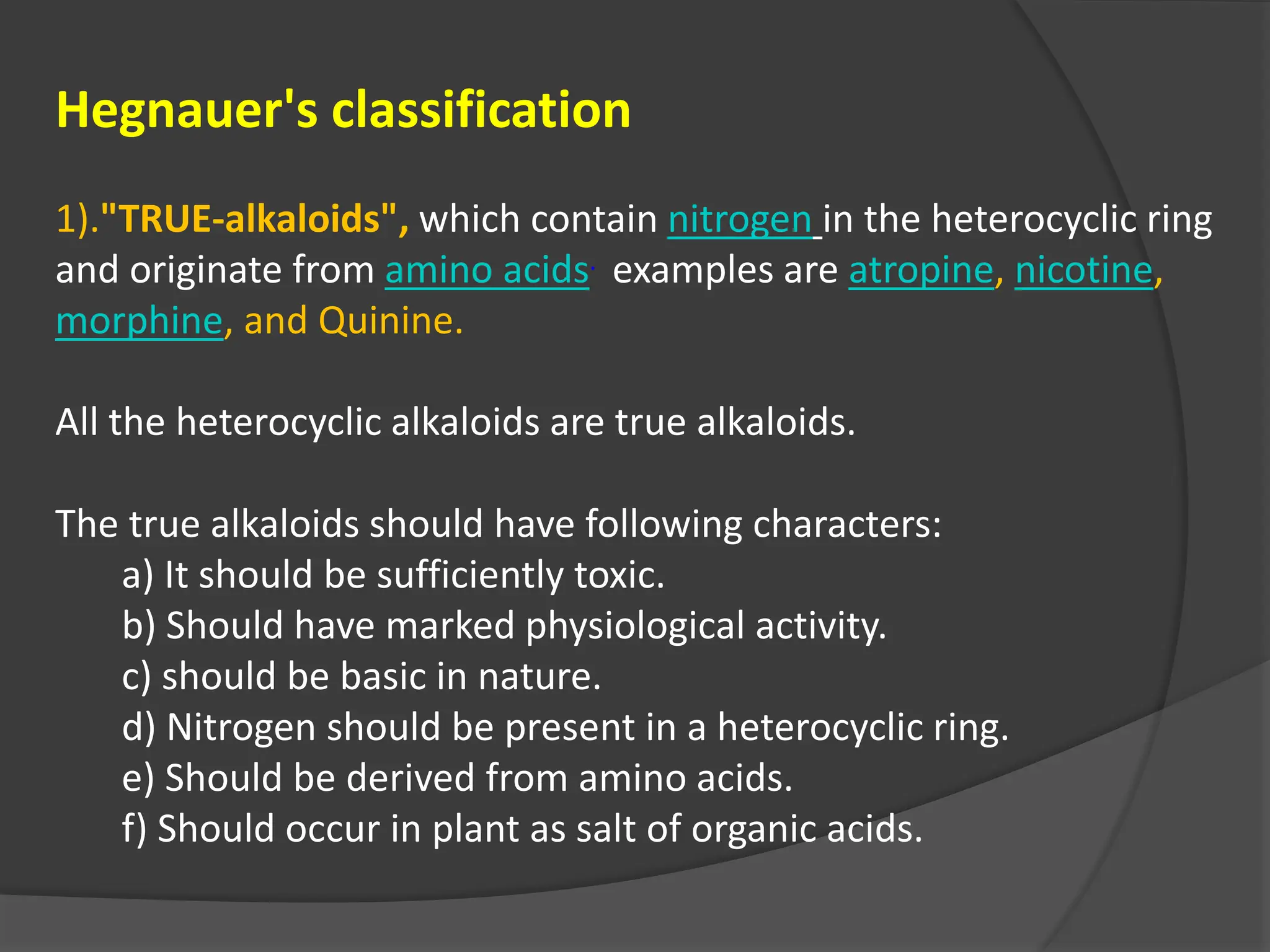
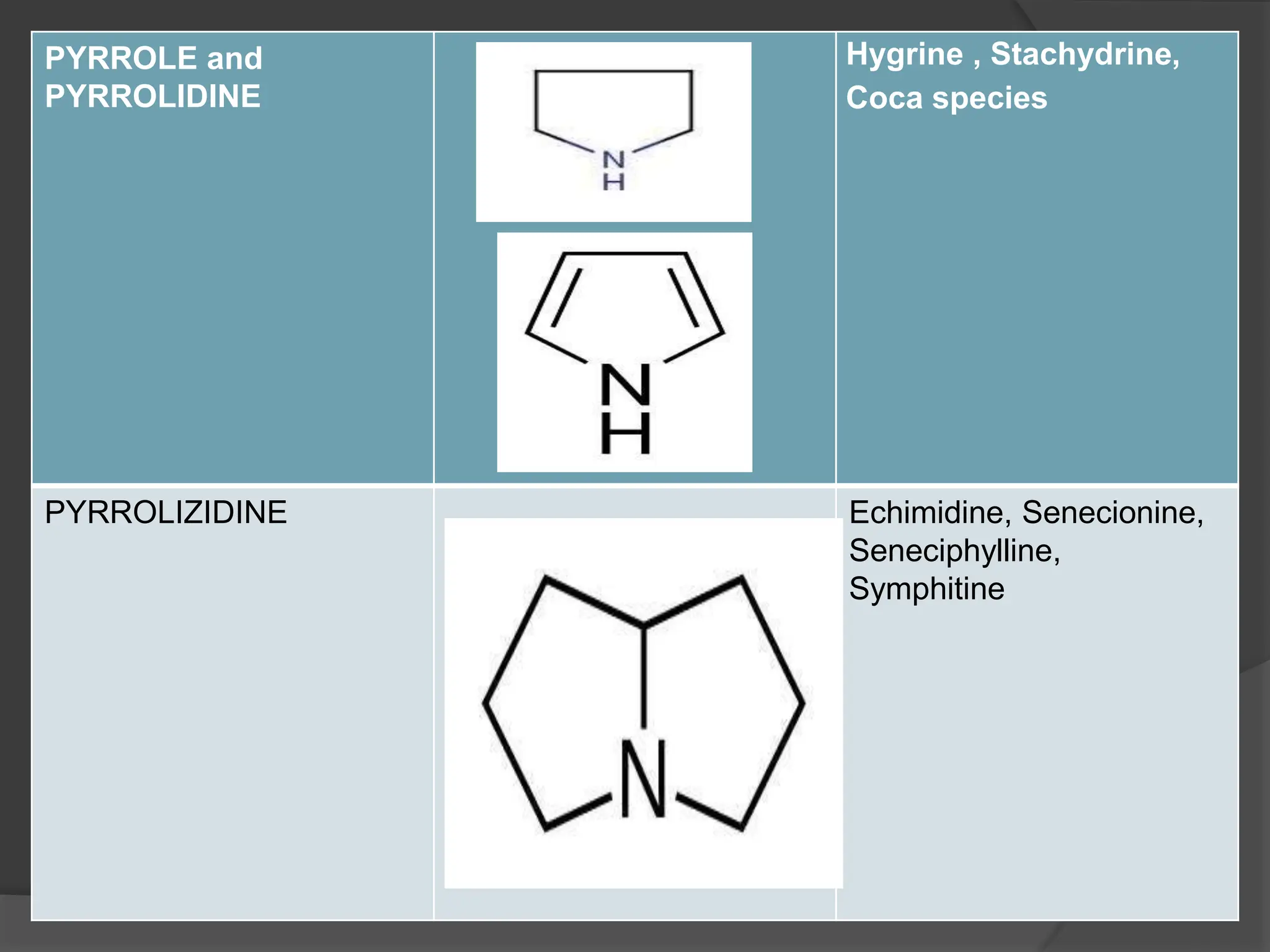
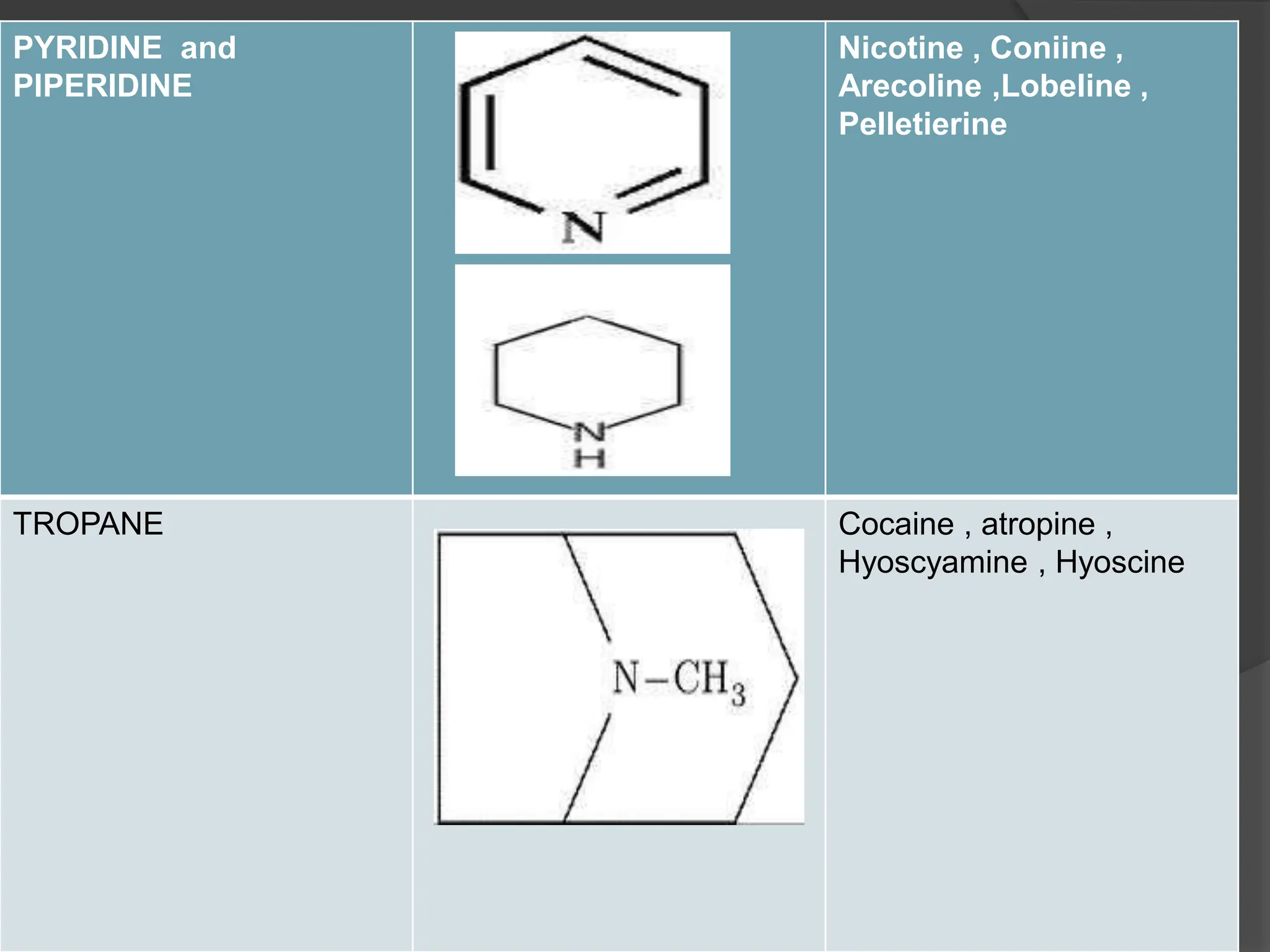
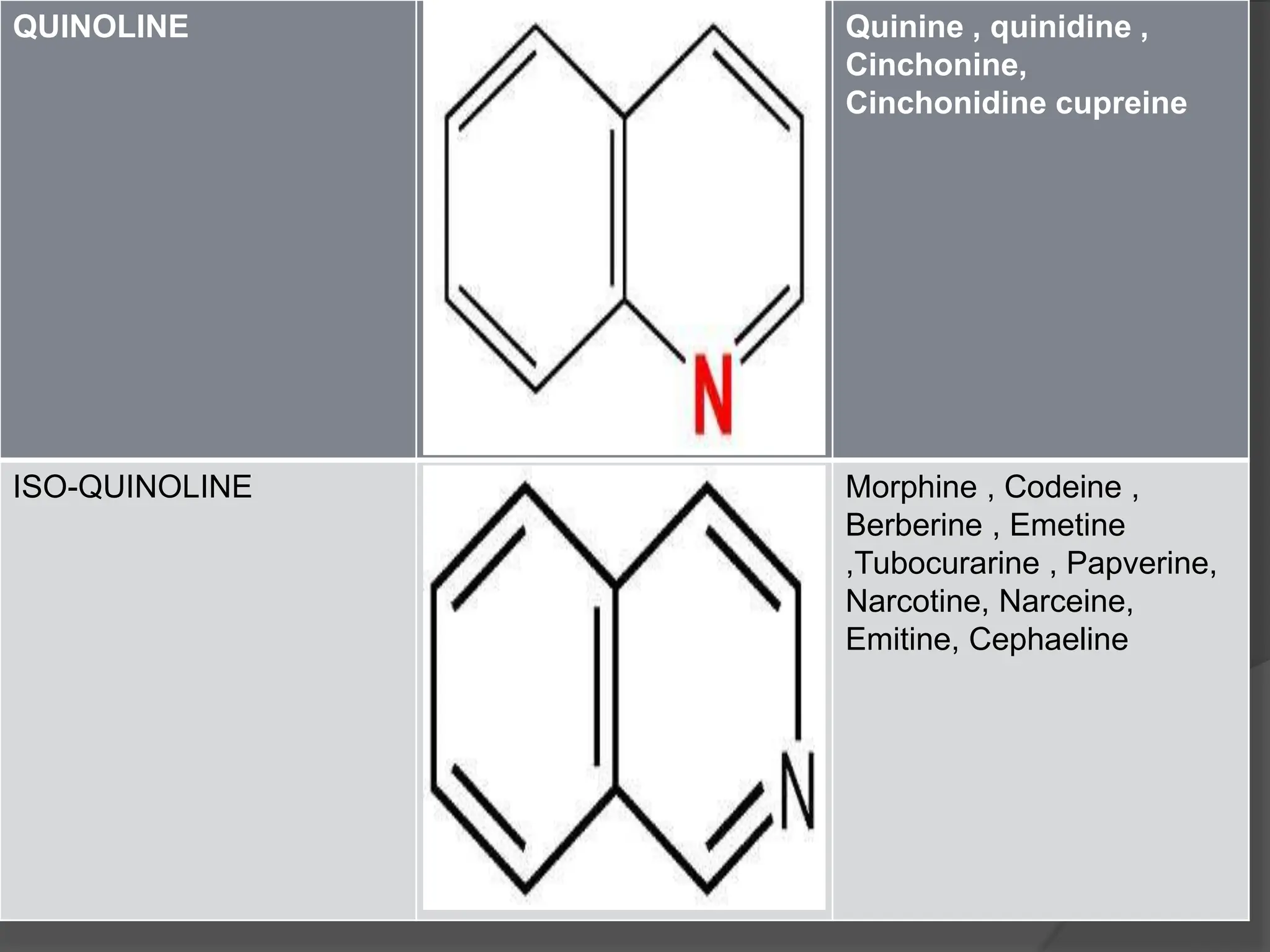
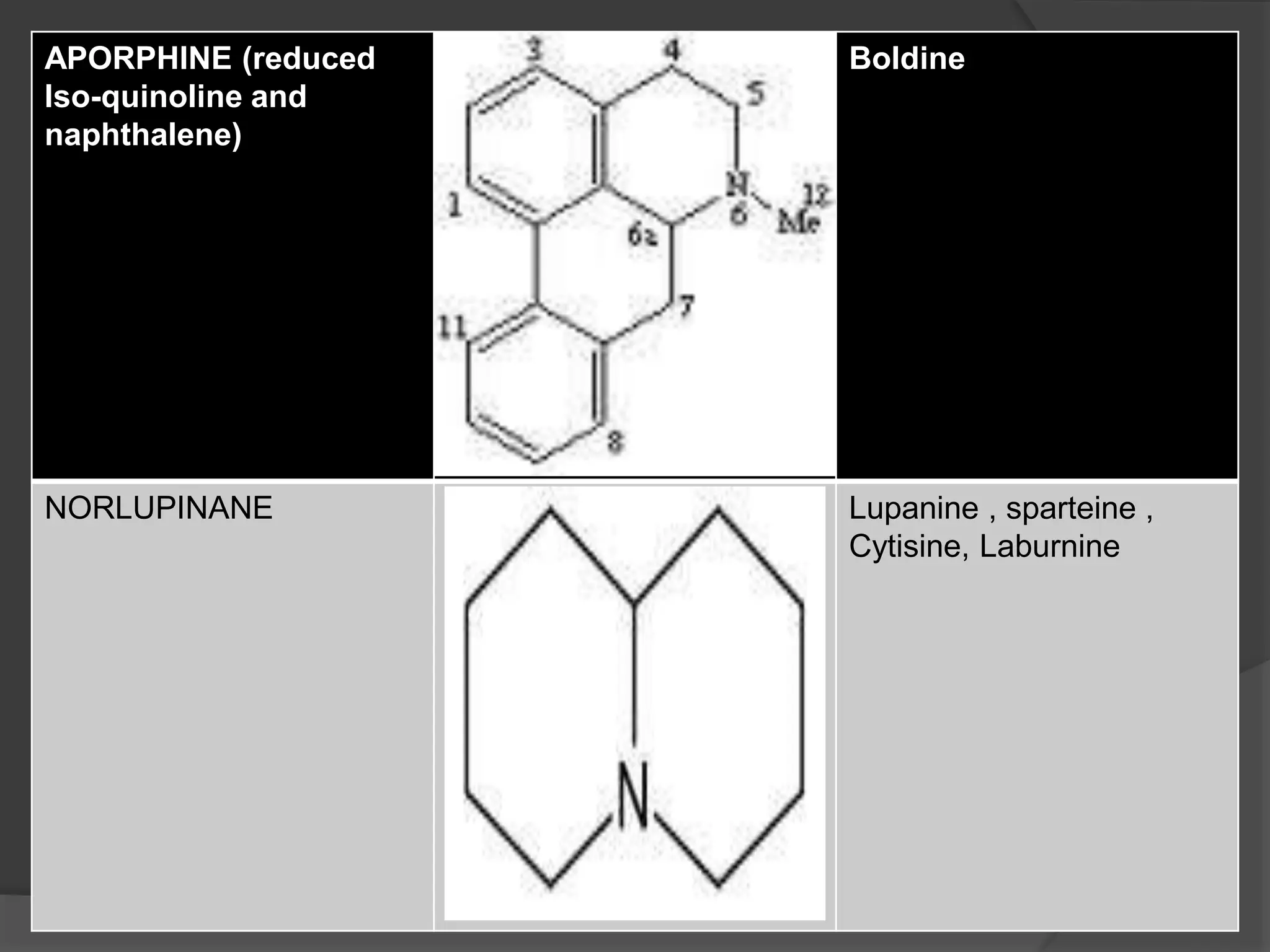
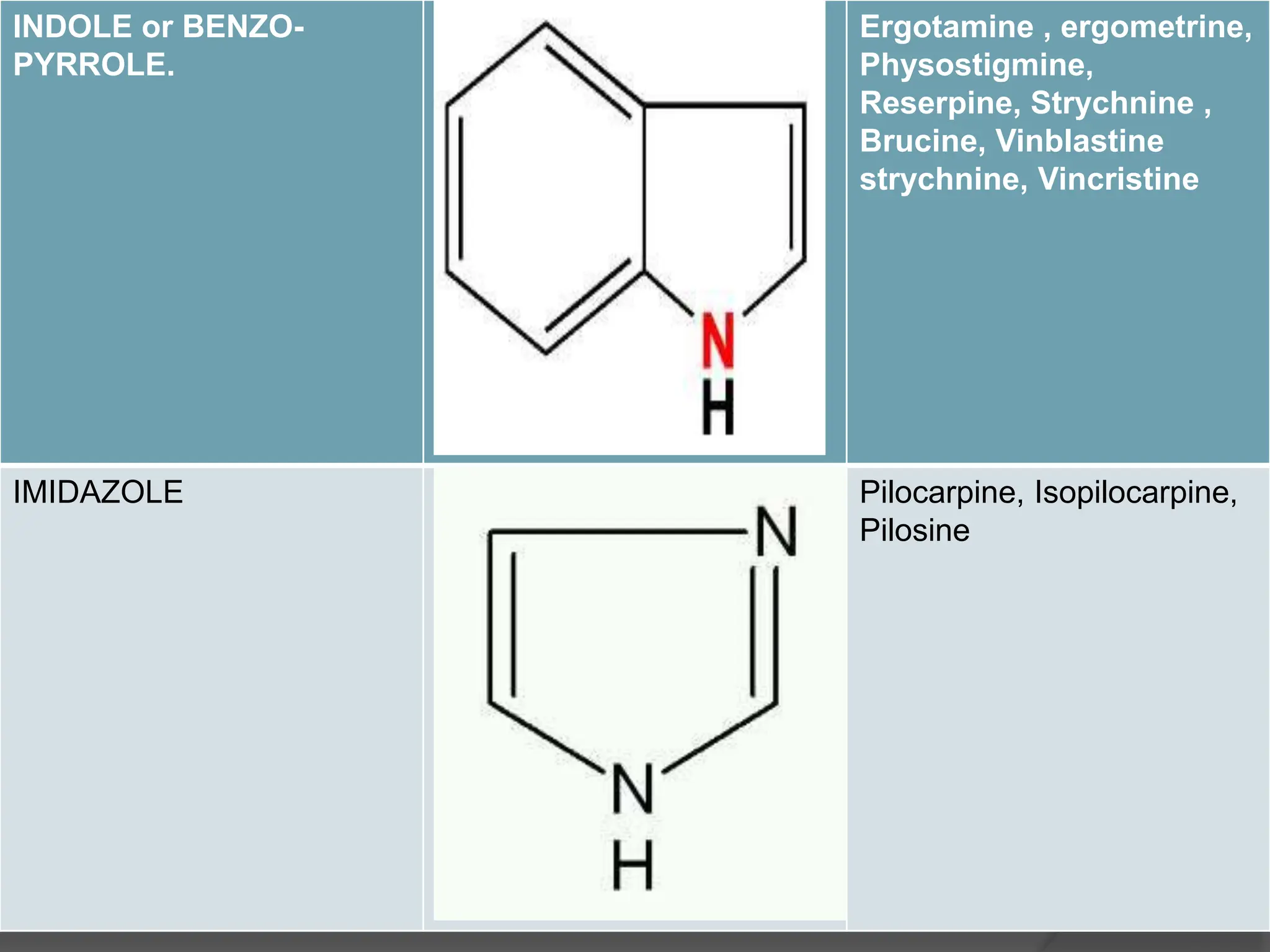
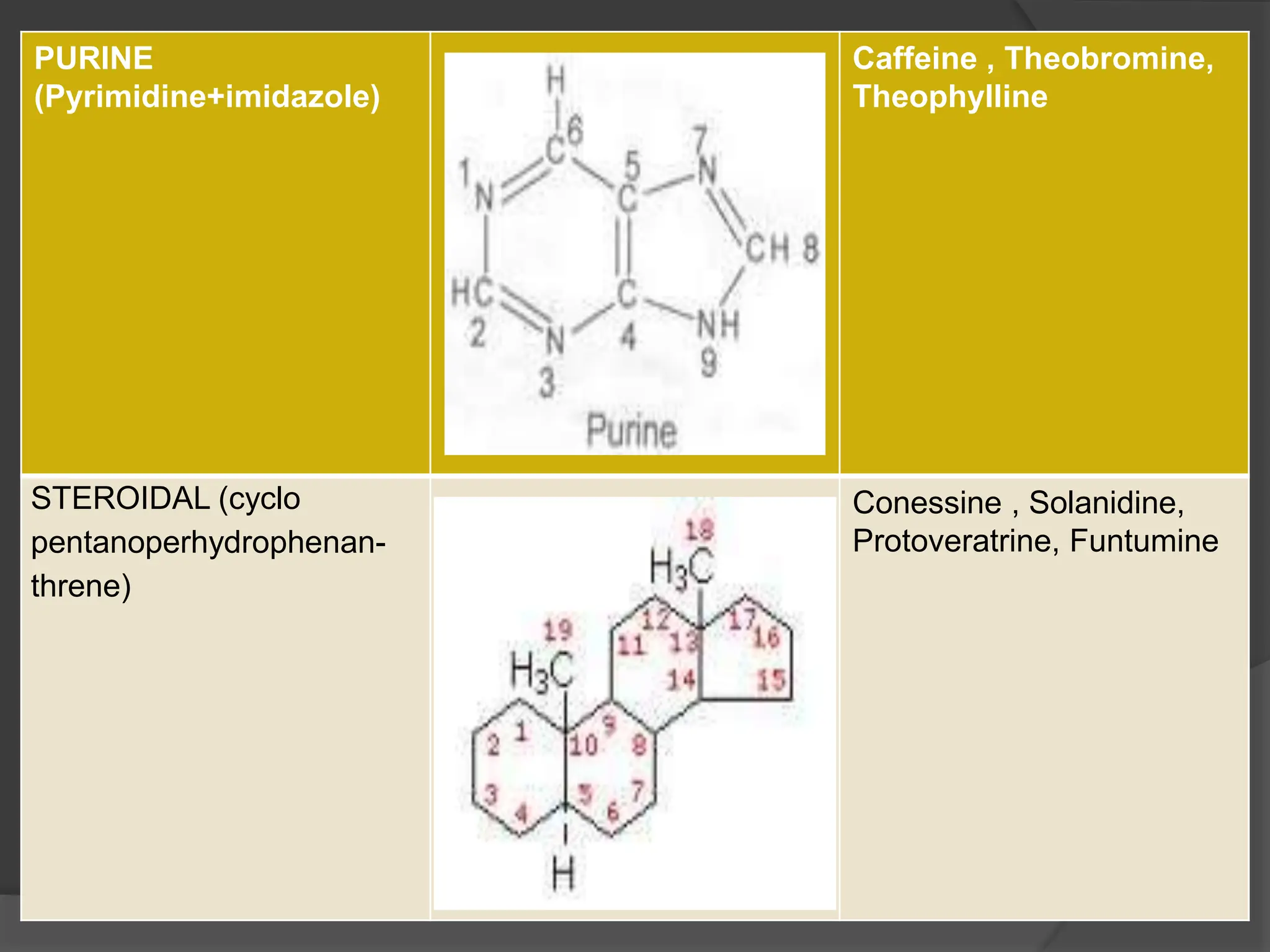
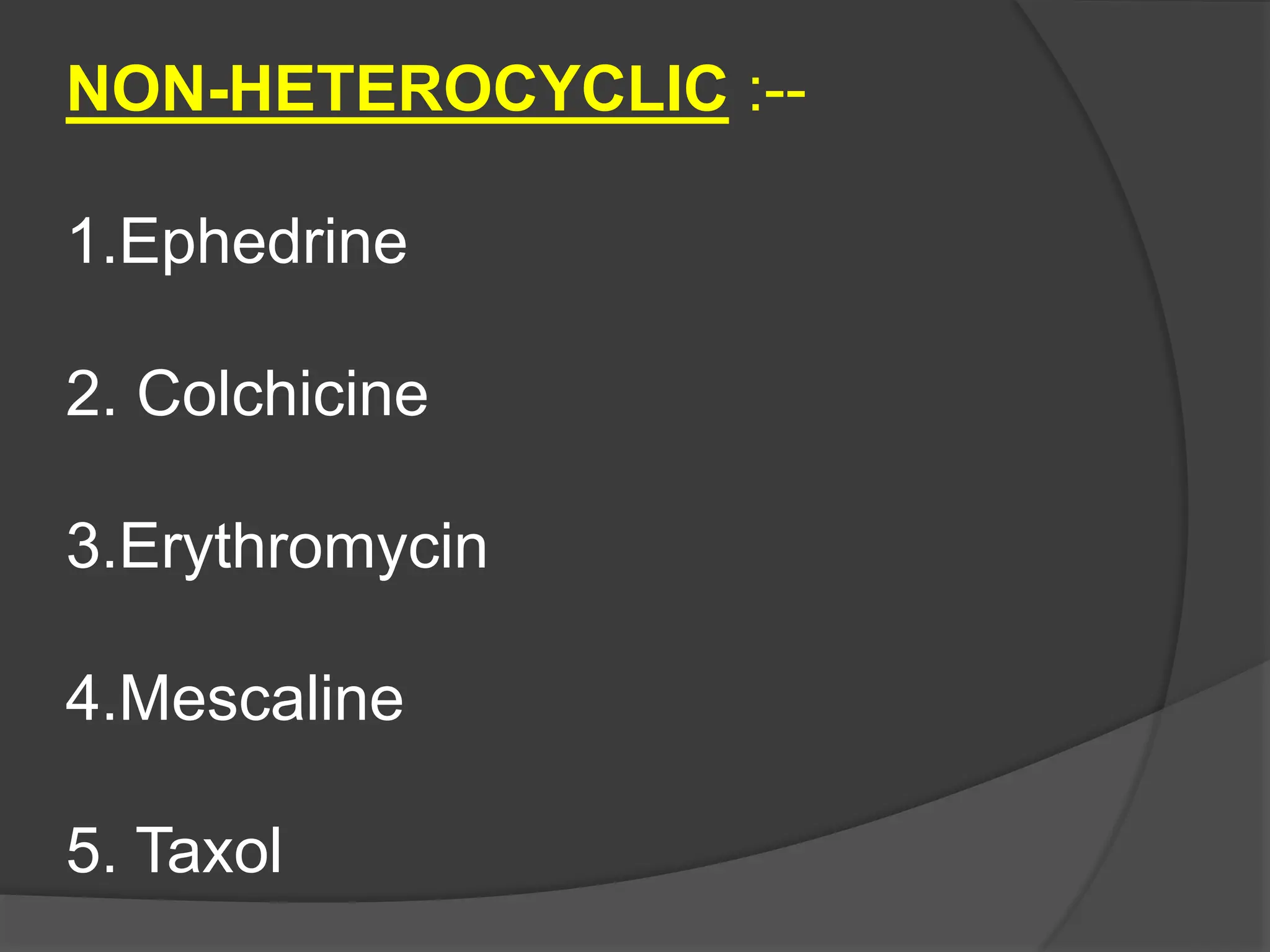
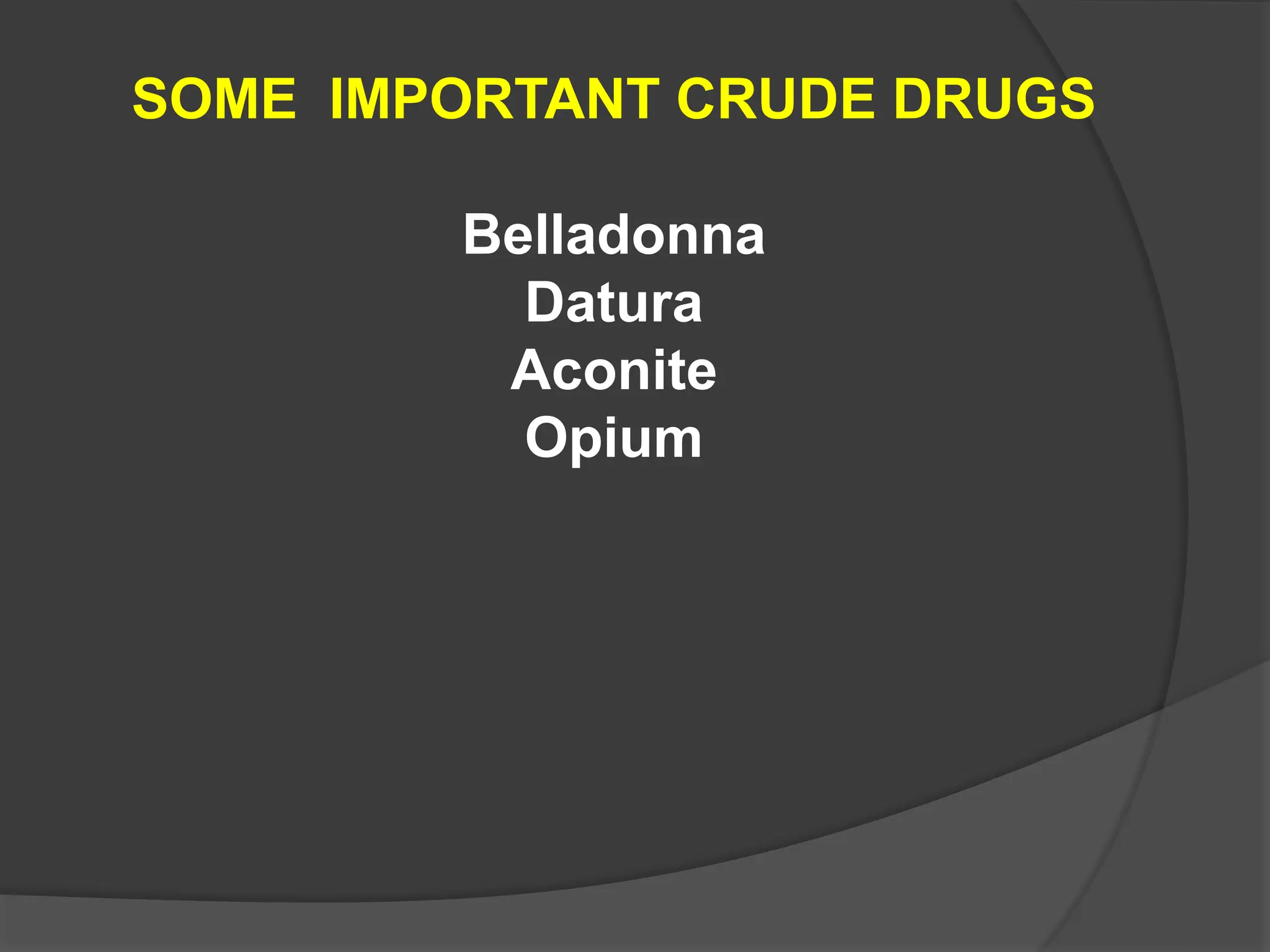
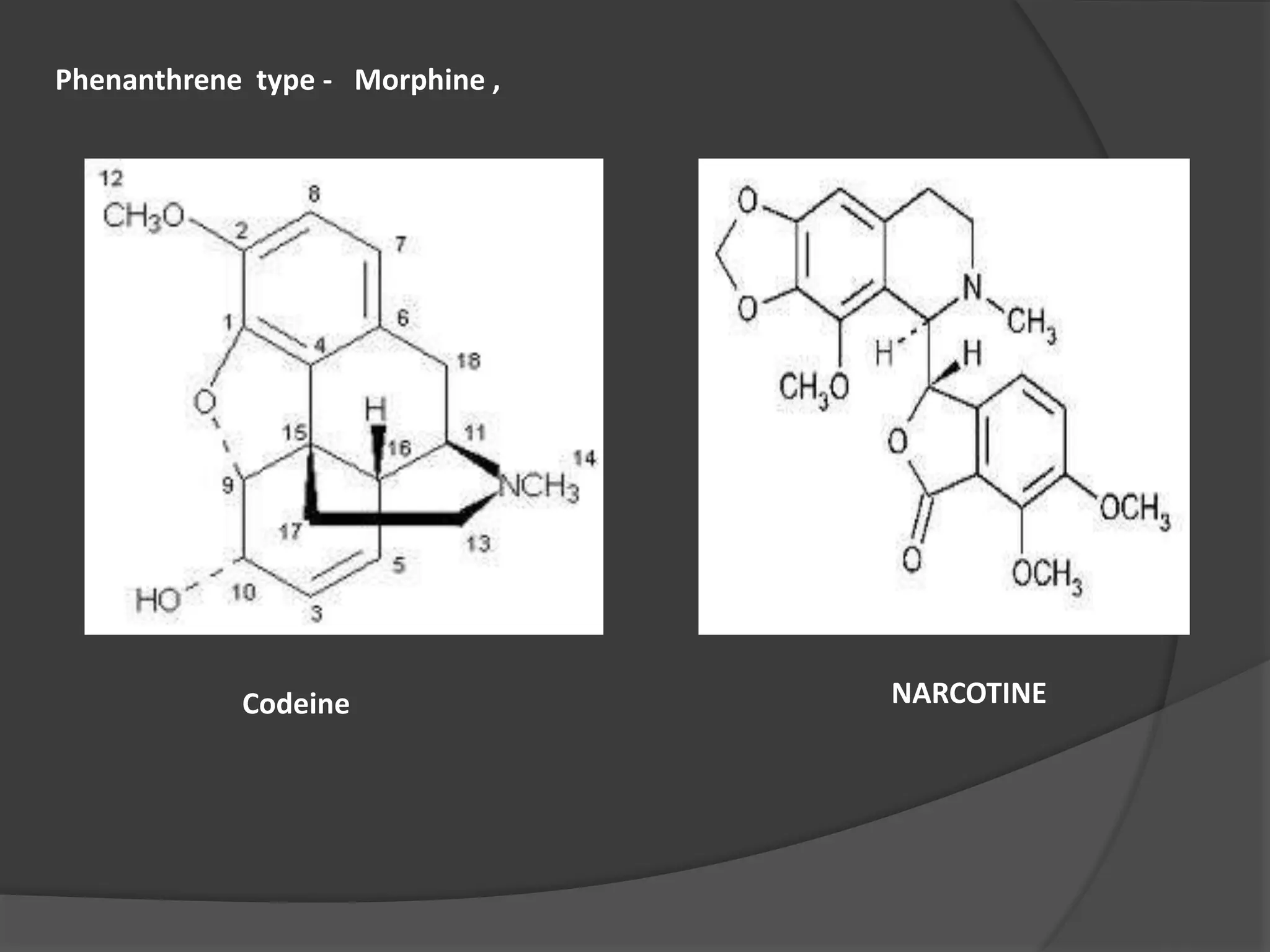
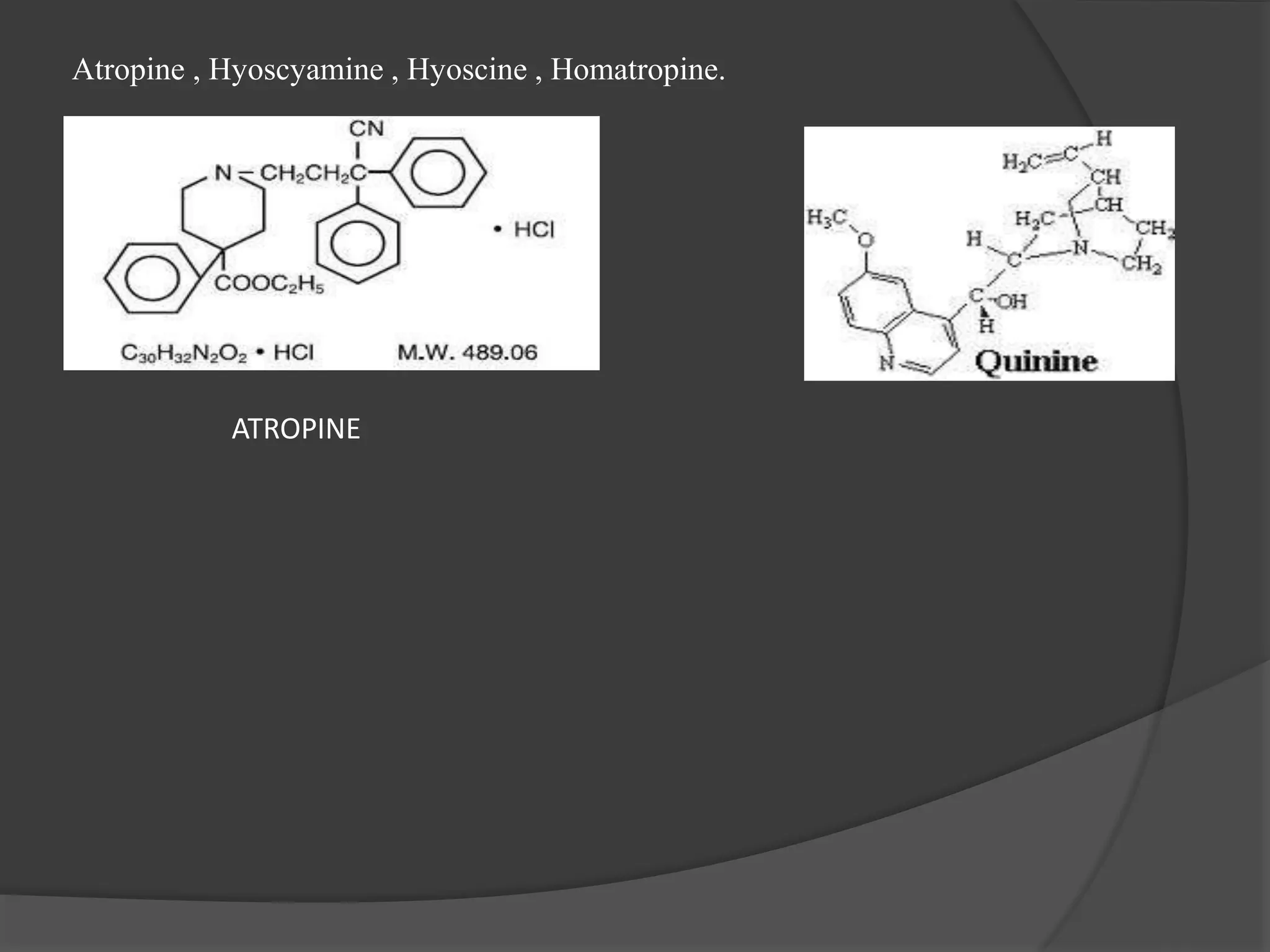
This document provides information on alkaloids. It begins by discussing the history of alkaloid use by ancient civilizations. It then defines alkaloids as basic nitrogenous compounds that contain heterocyclic rings and have physiological effects. The document outlines various alkaloid properties including solubility, reactions with acids and bases, and classification based on nitrogen content and ring structure. It also lists common plant sources of alkaloids and their medical and biological functions.