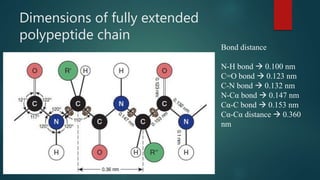
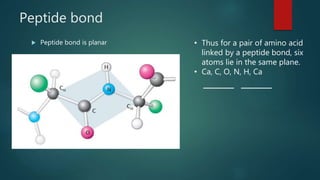
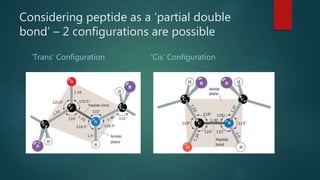
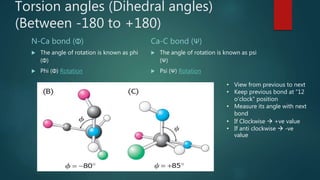
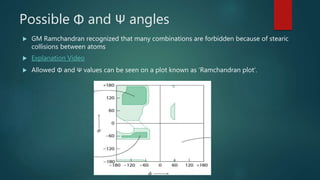
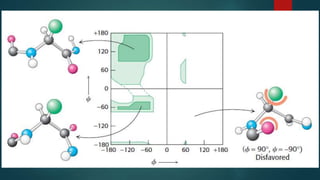
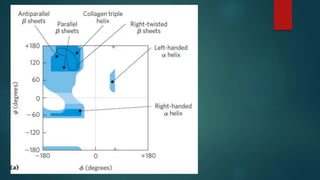
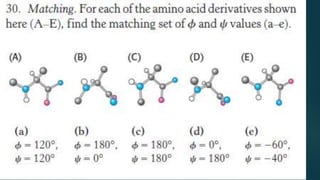
The document discusses the structure and properties of peptide bonds that link amino acids in proteins, including bond distances and configurations. It explains the significance of the 'trans' configuration, which is predominantly observed due to stearic clashes in the 'cis' configuration, especially in proline-containing linkages. The document also introduces the concept of torsion angles (phi and psi) and the Ramachandran plot, which illustrates allowed combinations of these angles based on steric constraints.