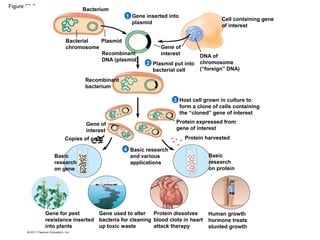
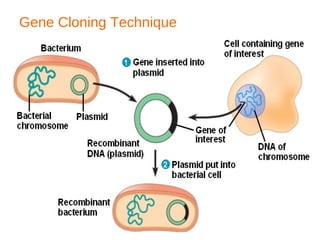
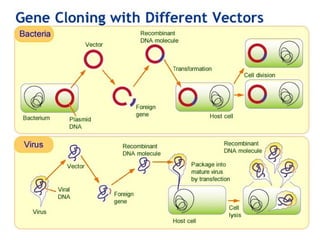
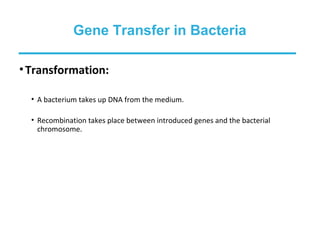
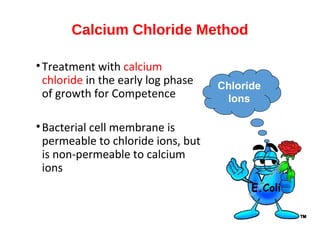
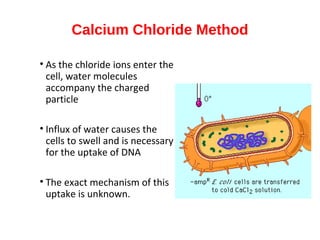
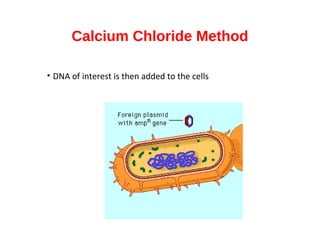
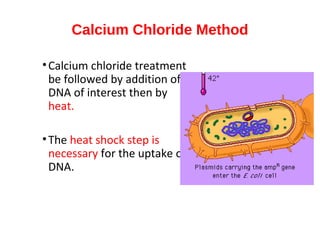
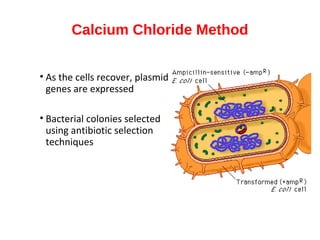
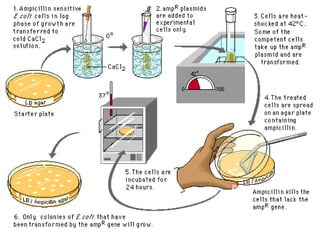
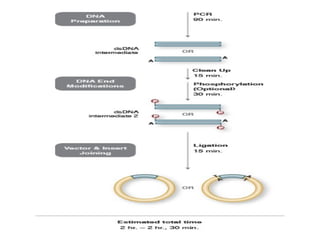
Gene cloning involves inserting foreign DNA into plasmids which are then propagated in bacterial cells to produce multiple copies of a gene. Gene manipulation refers to the formation of new combinations of heritable material in host organisms using vectors. Methods like calcium chloride treatment and PCR cloning provide efficient techniques for gene cloning, though they come with certain limitations such as higher costs and restricted vector options.