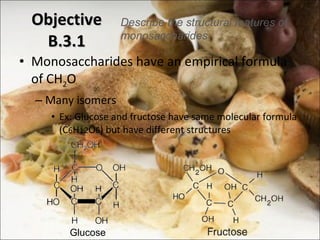
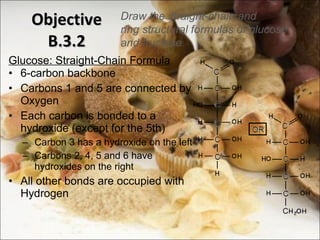
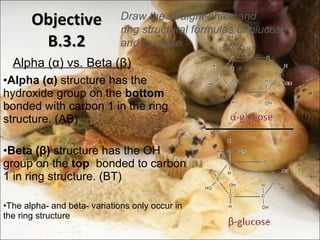
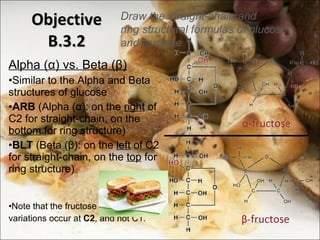
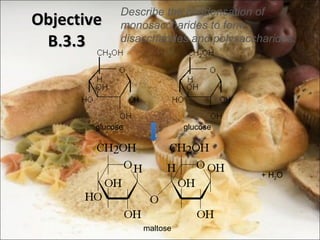
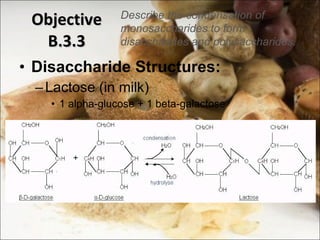
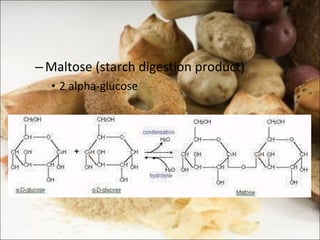
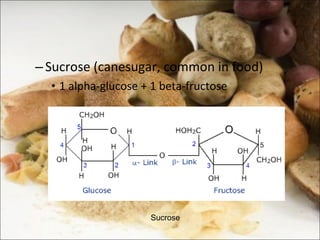
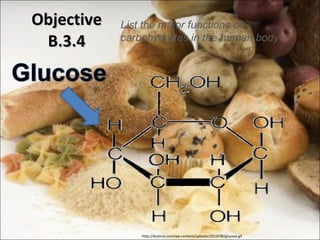
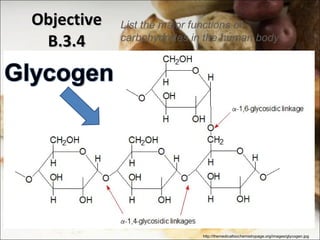
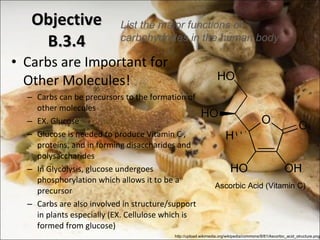
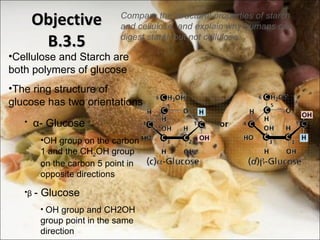
This document outlines objectives related to carbohydrate structure and function. It describes the structural features of monosaccharides like glucose and fructose, how they combine to form disaccharides and polysaccharides, and examples like starch, glycogen and cellulose. It also summarizes the major functions of carbohydrates in the human body like energy storage and providing precursor molecules, and why humans can digest starch but not cellulose due to structural differences. Finally, it defines dietary fiber and its importance for health.