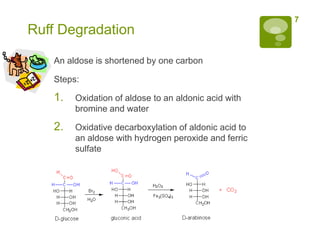
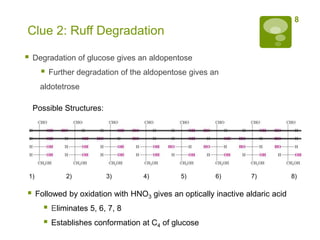
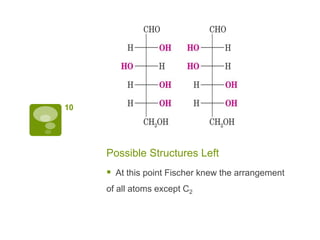
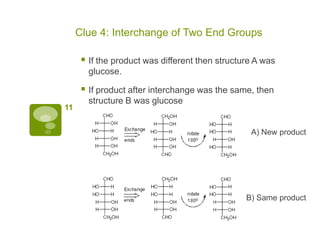
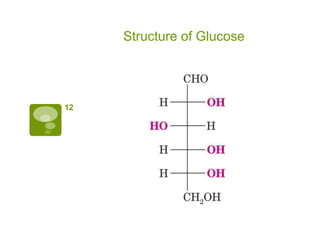
The document summarizes the process by which Emil Fischer determined the structure of glucose in the late 19th century. [1] Glucose has 4 chiral centers, allowing for 16 possible stereoisomers. [2] Through oxidation and degradation experiments, Fischer was able to determine the stereochemistry at carbons 3, 4, and 5, leaving only carbon 2 undefined. [3] An experiment interchanging end groups showed that glucose has structure A, with stereochemistry now fully characterized.