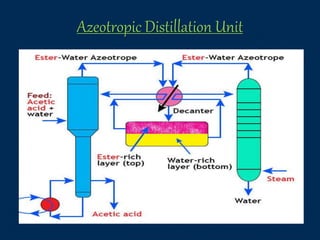
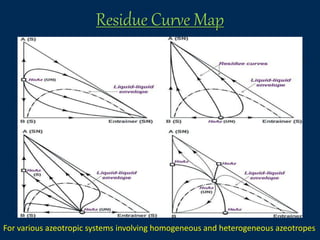
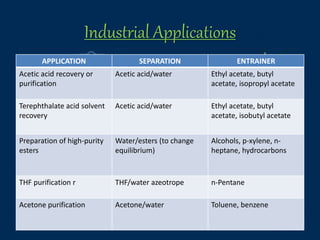
This document provides an overview of azeotropic distillation. It begins with an introduction that defines azeotropic distillation as a process where an entrainer is added to a feed mixture to form an azeotrope that can be separated. The document then discusses the working principle, provides examples of residue curve maps, and outlines considerations for process design and simulation. Finally, it discusses several industrial applications of azeotropic distillation, including alcohol dehydration, acetic acid dehydration, and ester production, before concluding and listing references.