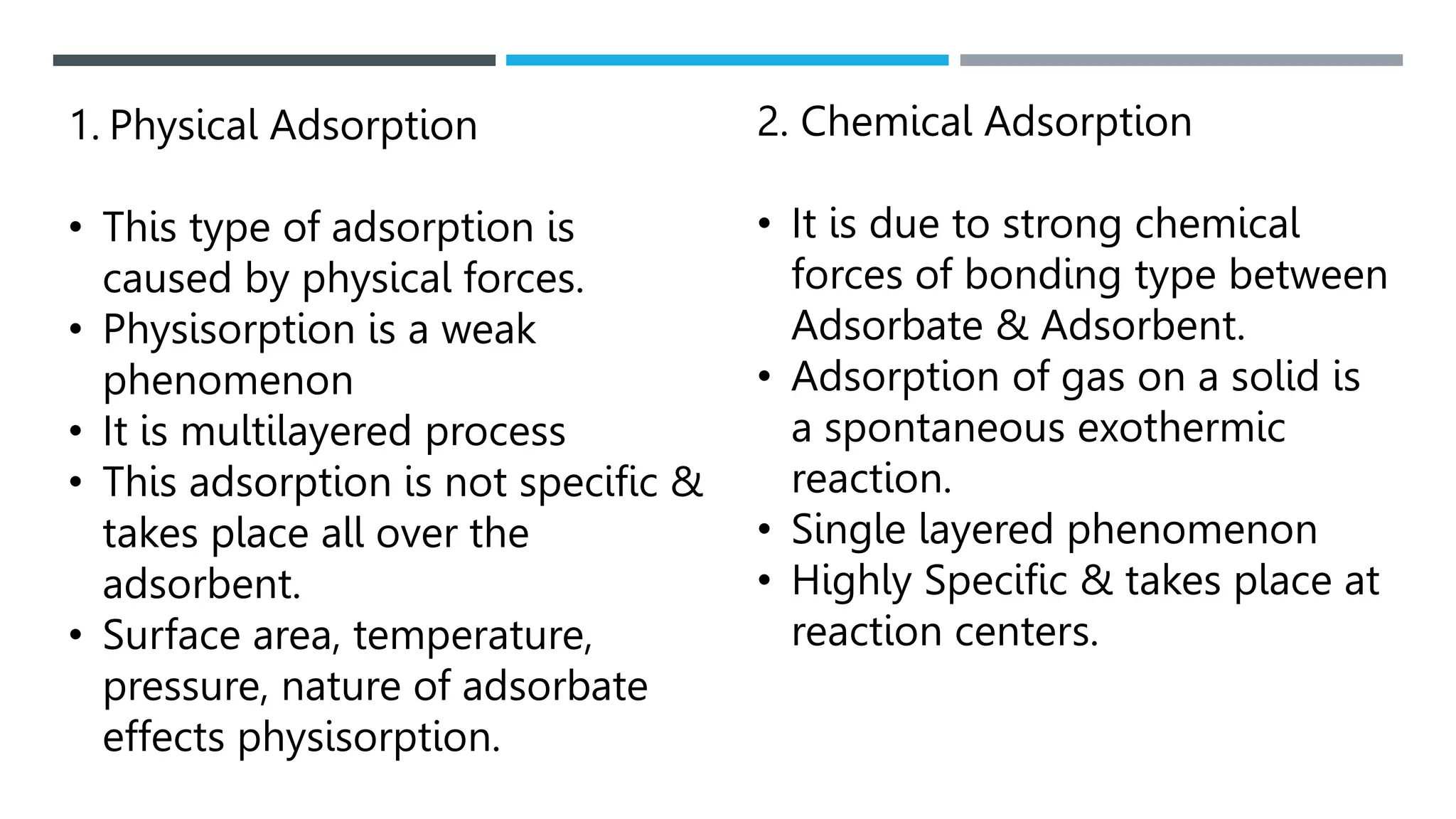
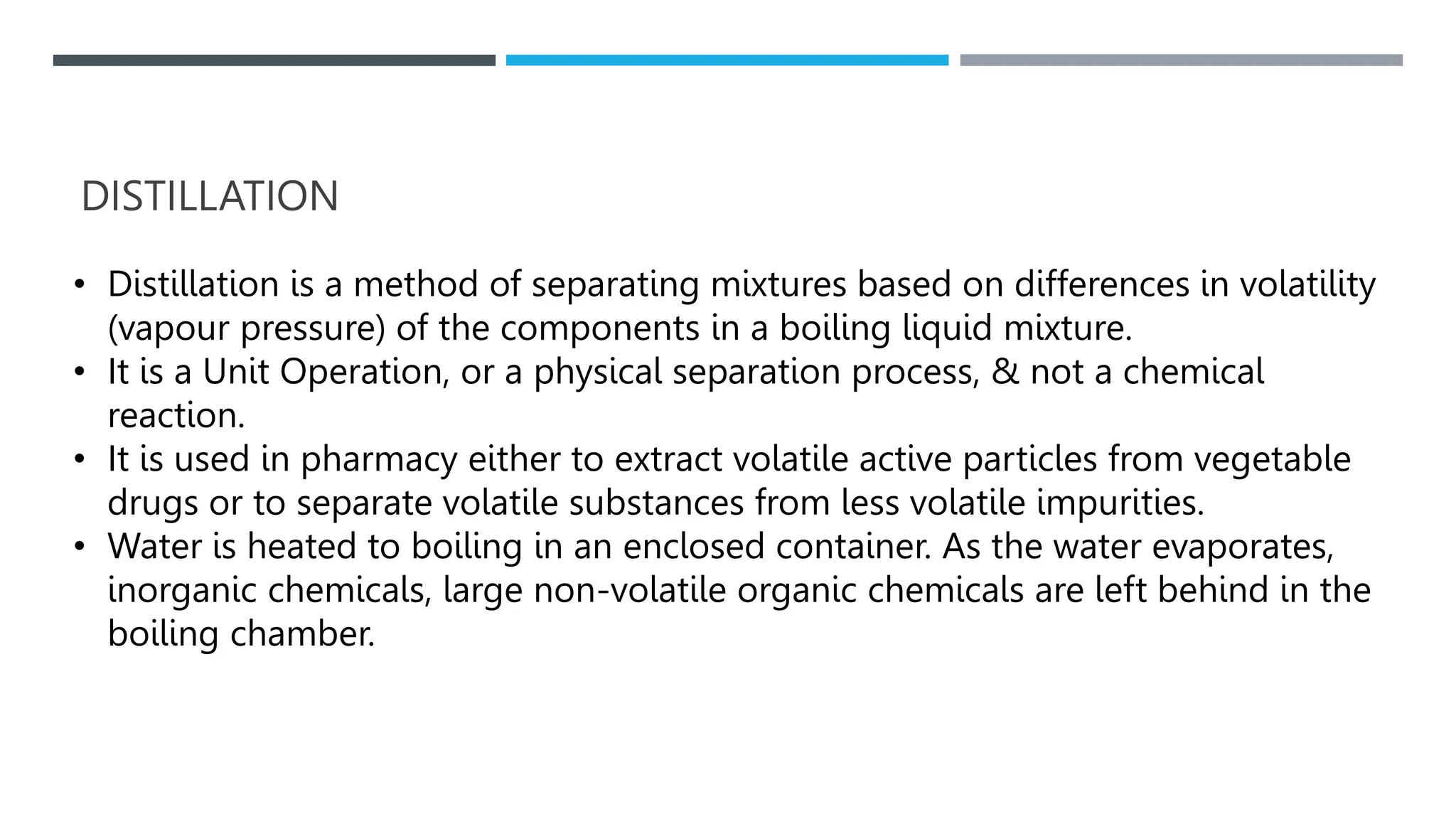
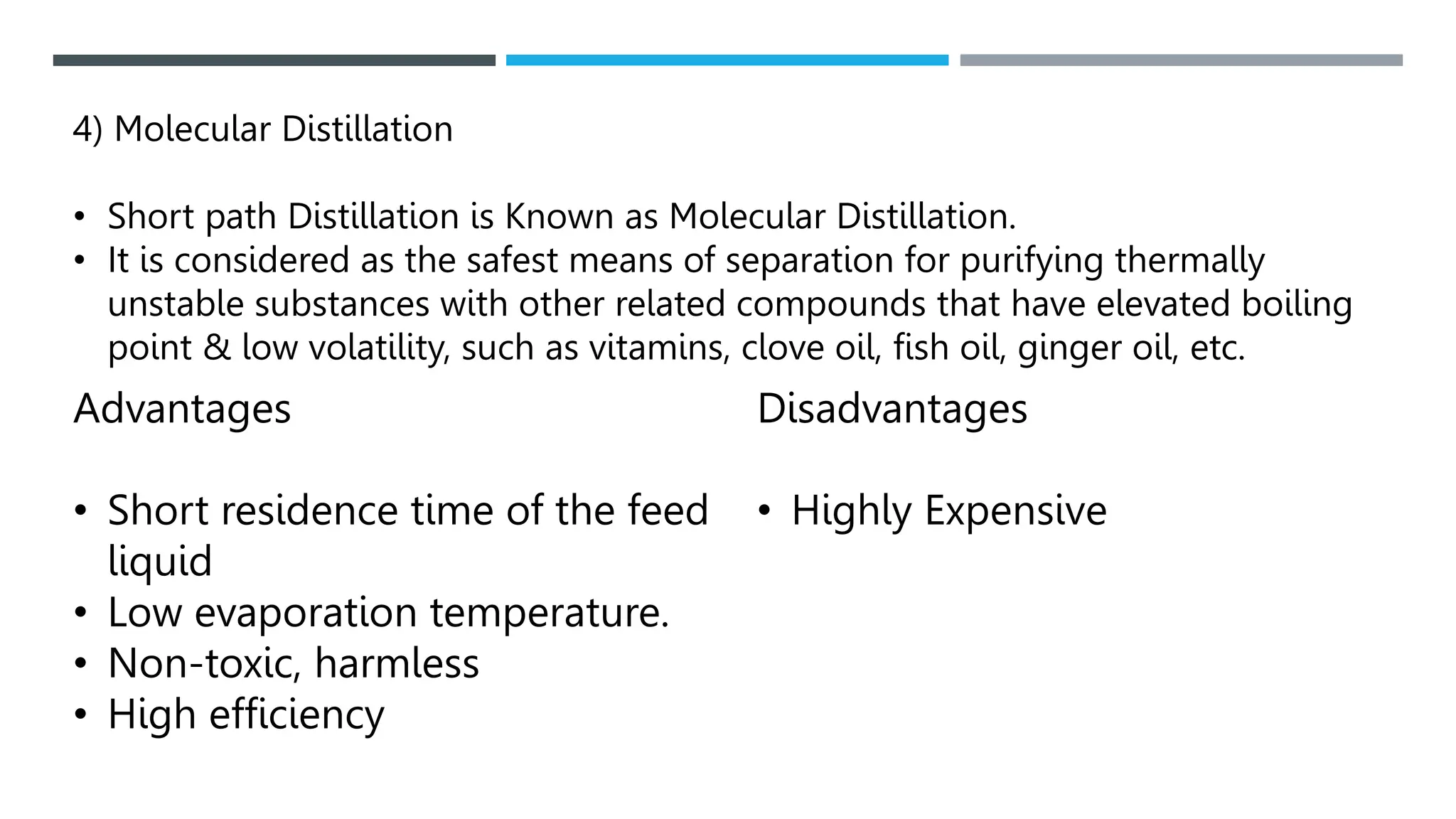
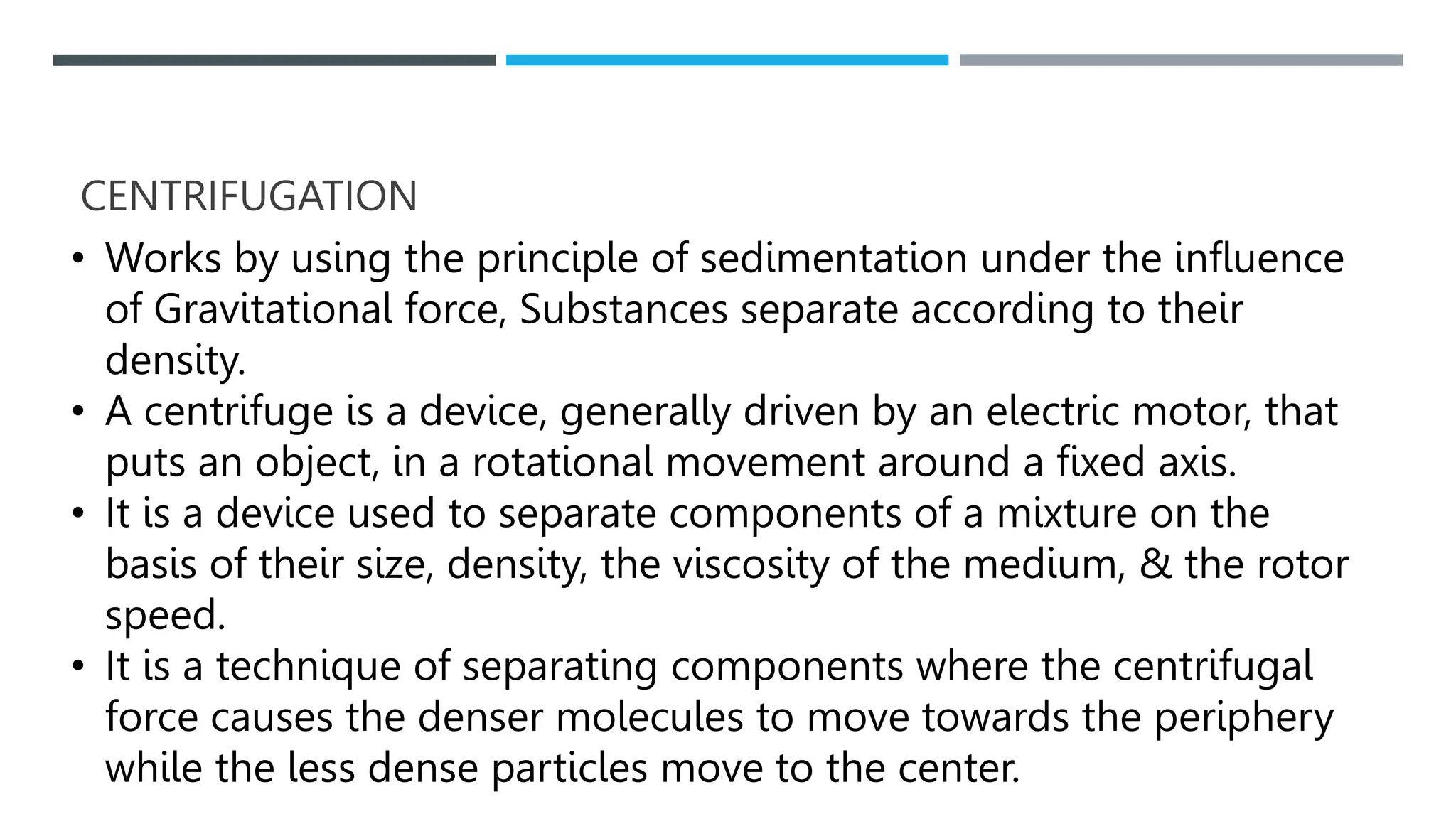
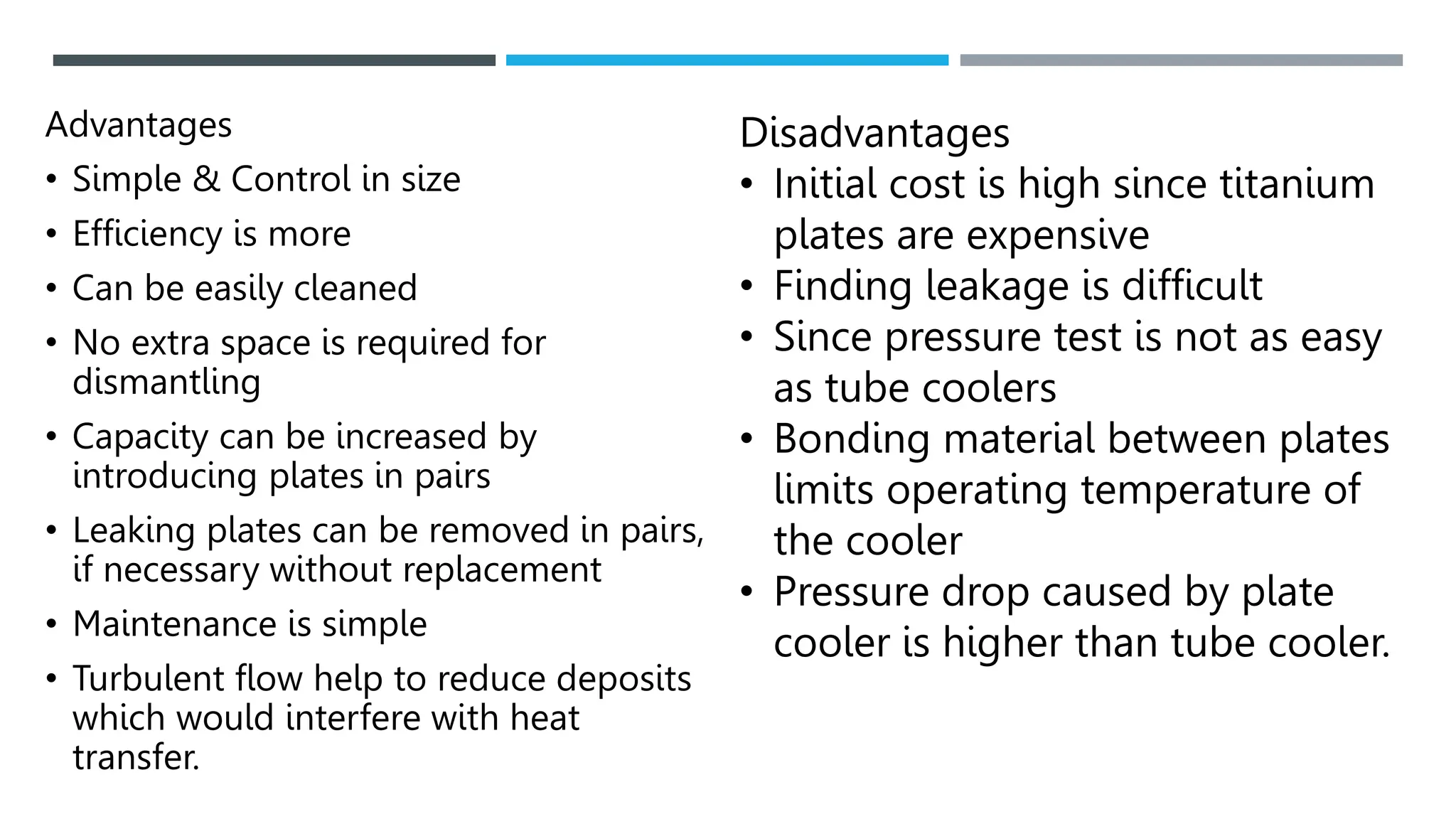
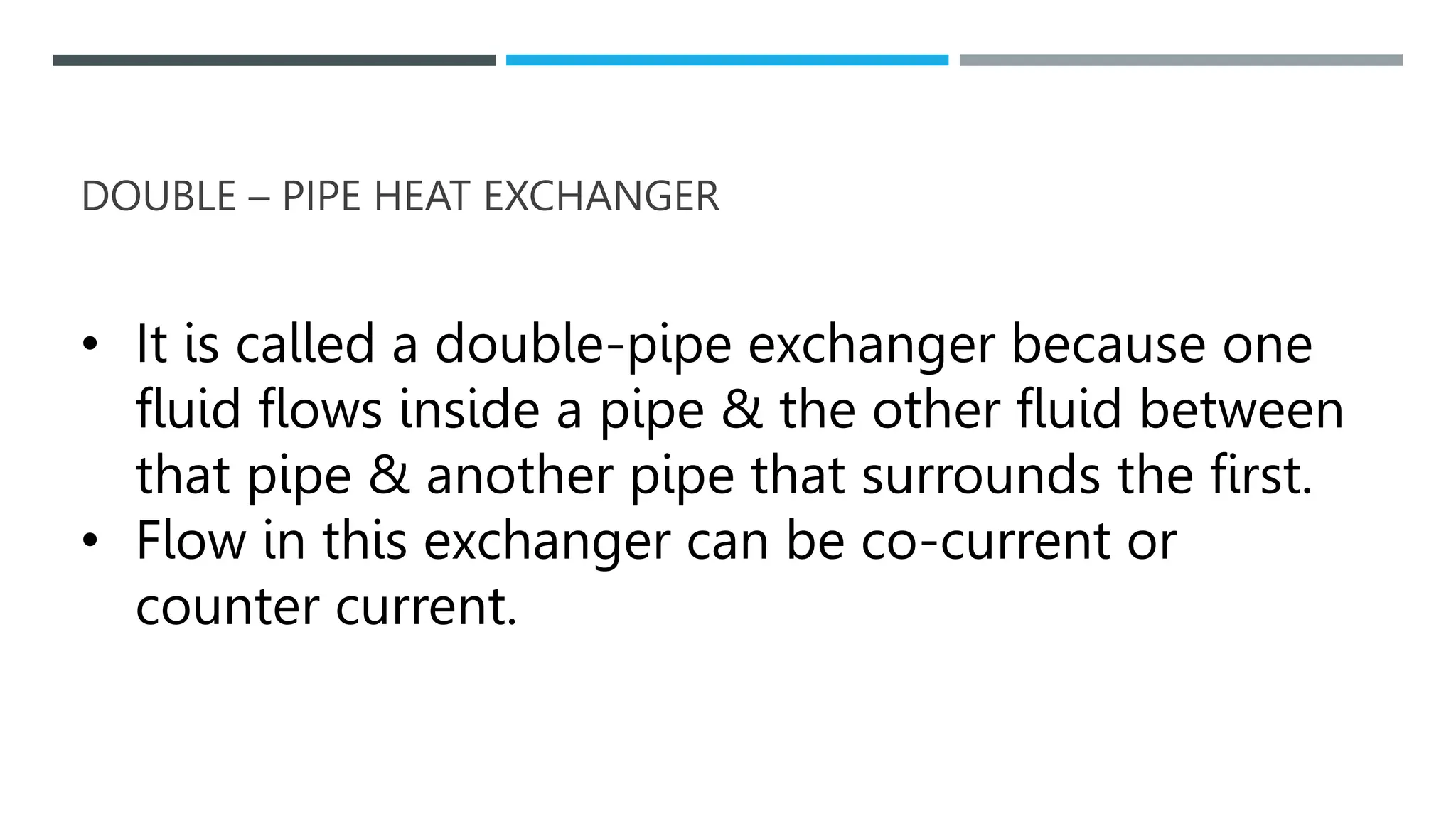
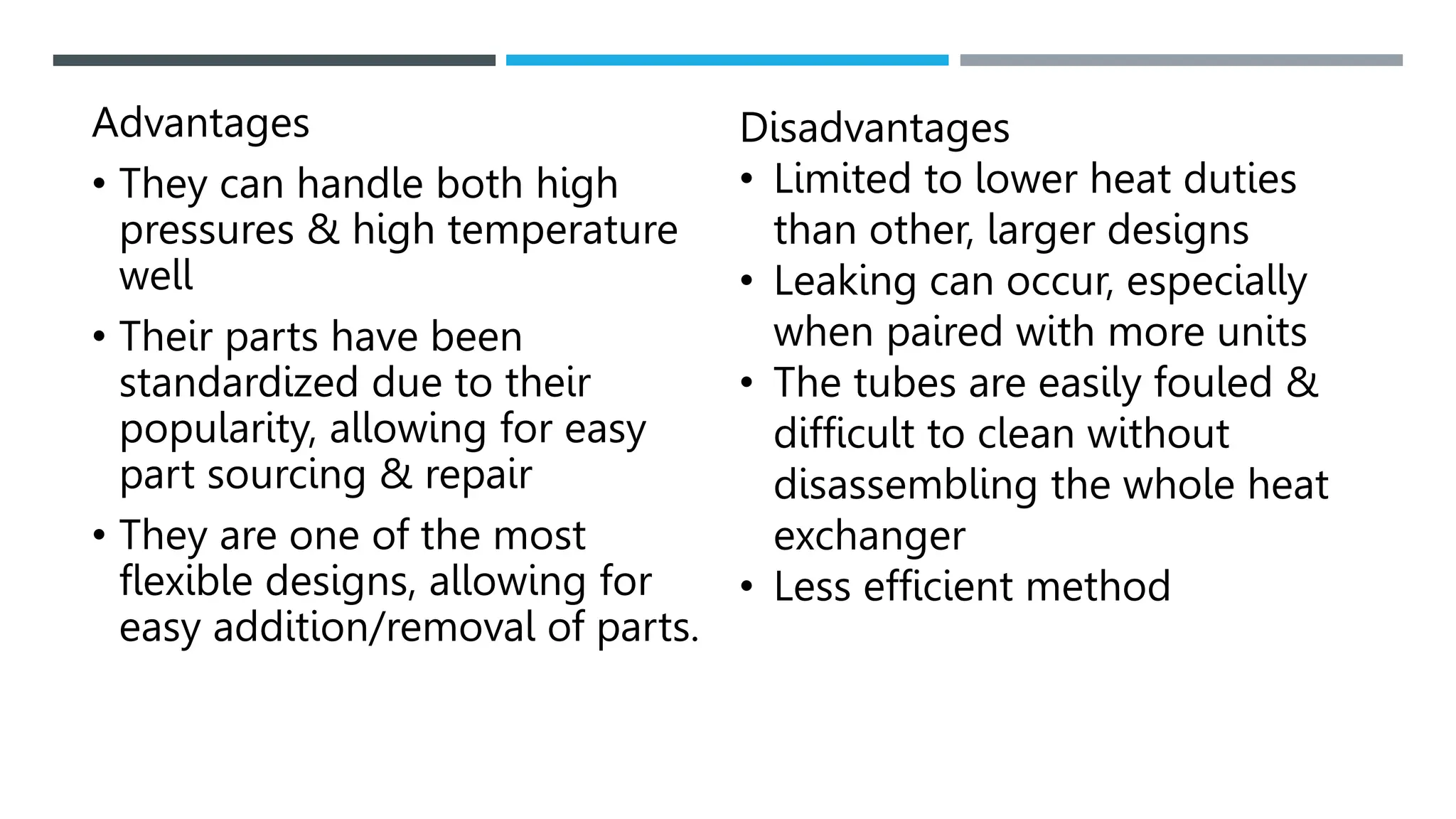
Unit operations in chemical engineering encompass various physical processes used to alter the state of materials without chemical reactions, including absorption, adsorption, distillation, and drying. Each process involves specific mechanisms and techniques, such as physical or chemical absorption, and has different types, advantages, and disadvantages, like simple and fractional distillation. The document outlines these operations, detailing principles, methods, and applications relevant to manufacturing and chemical processing.