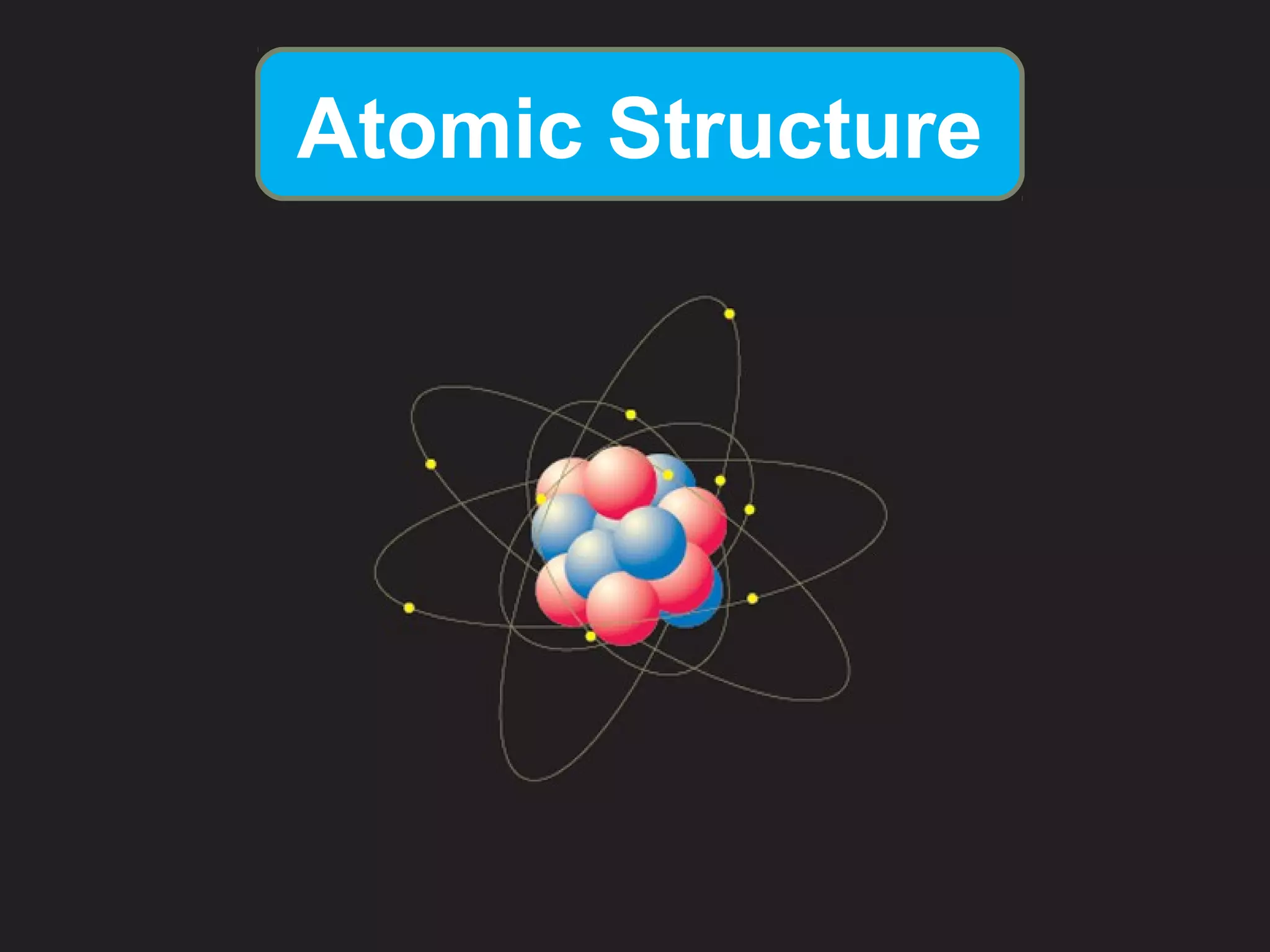
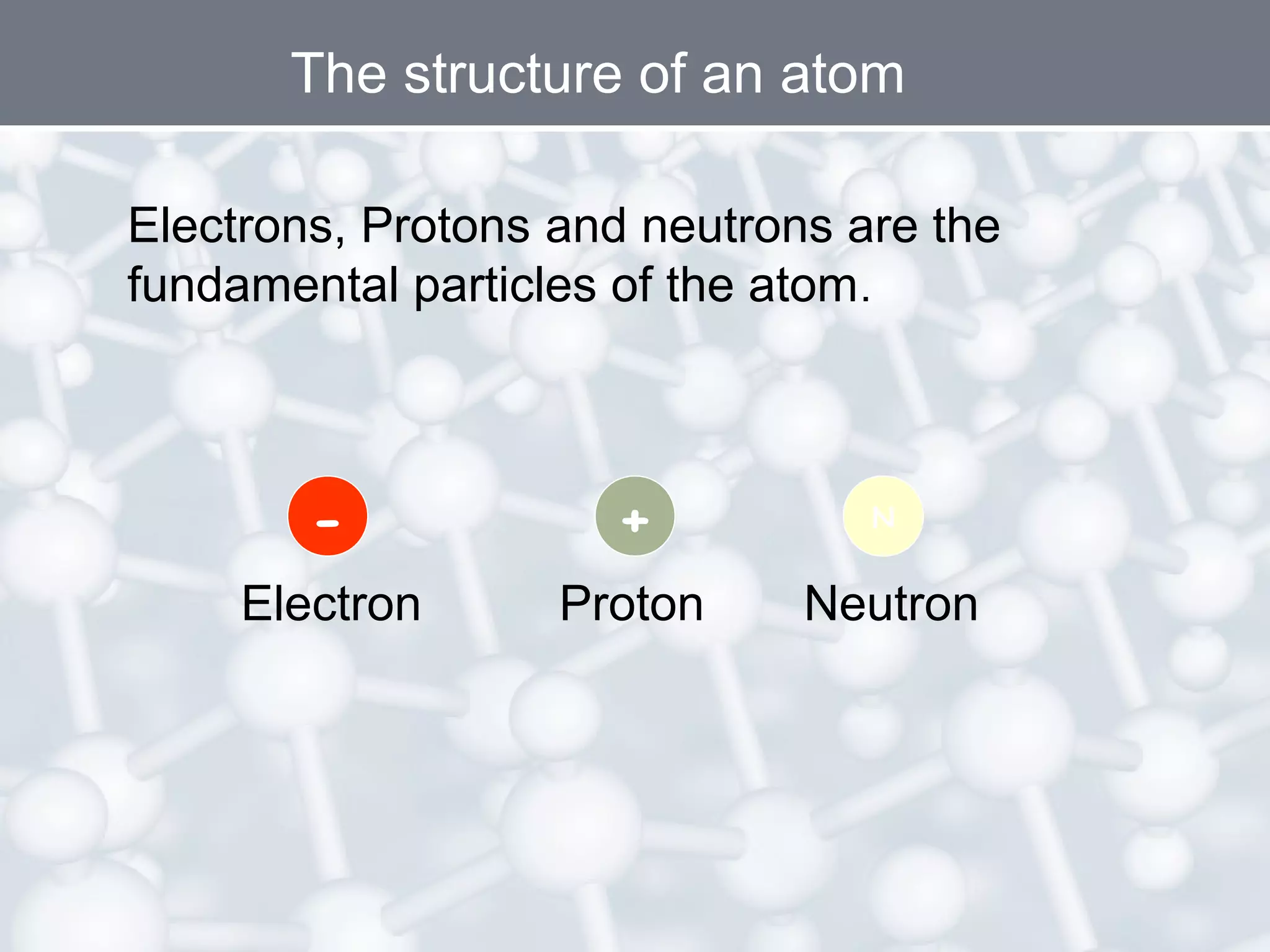
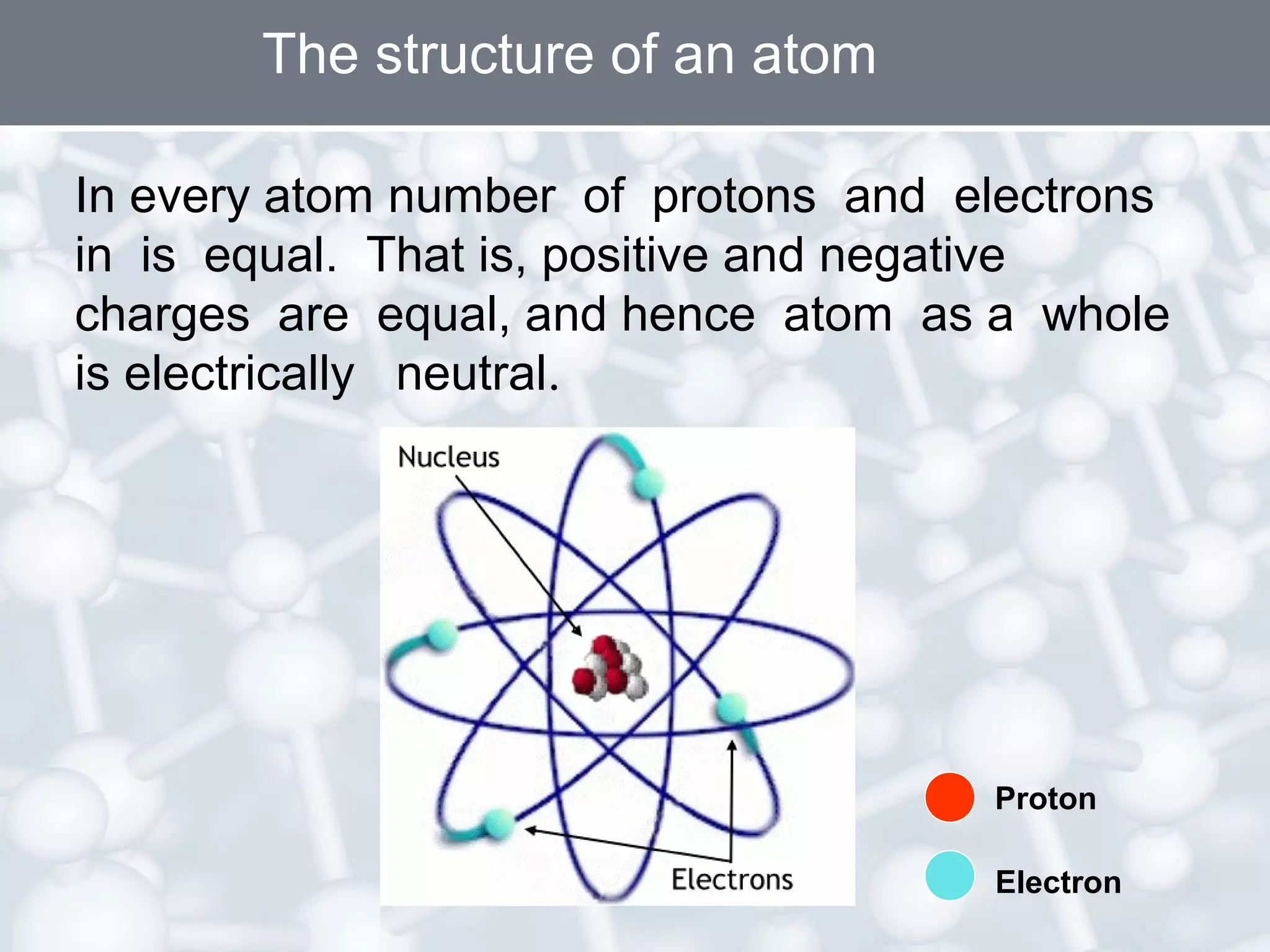
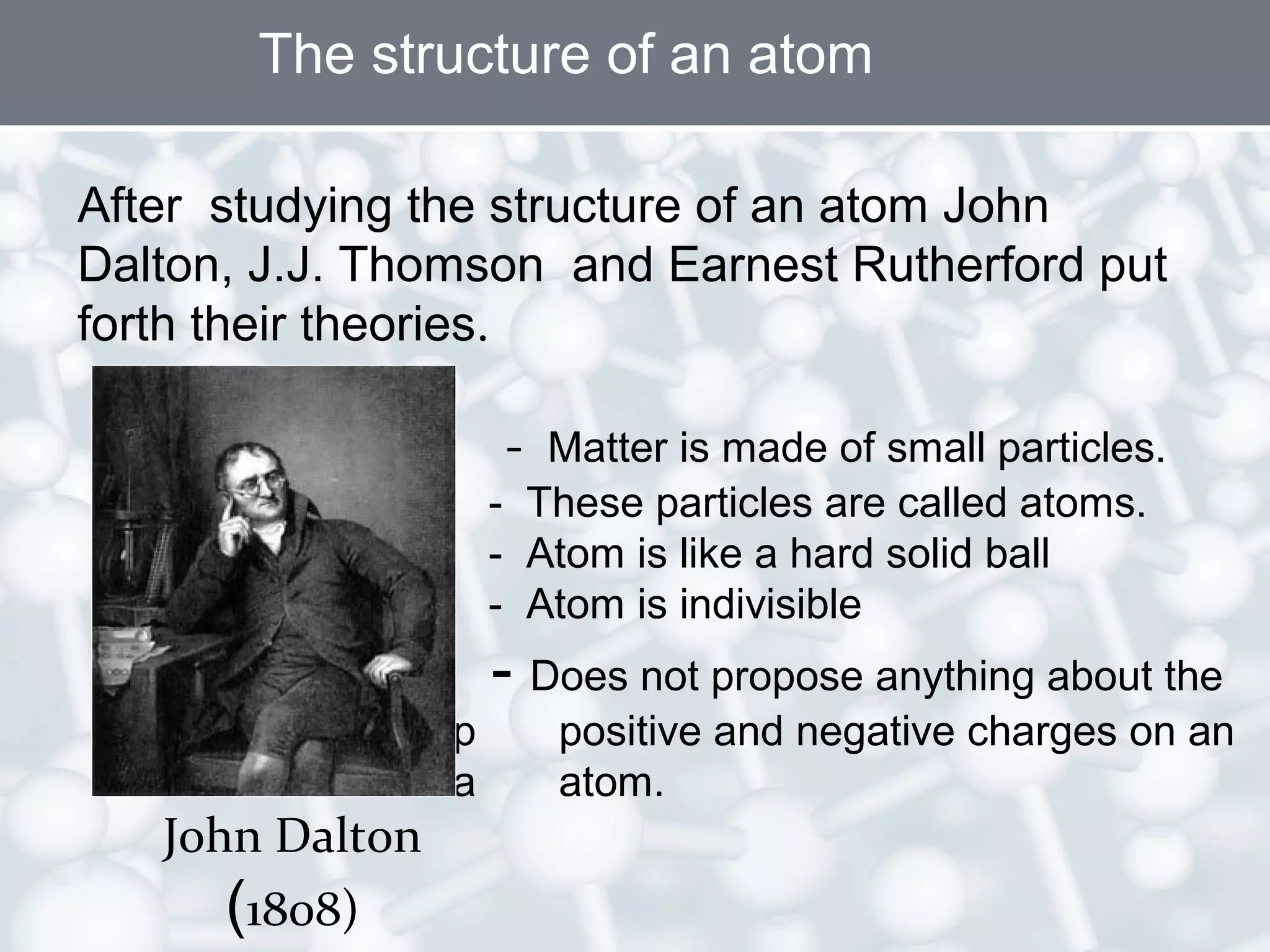
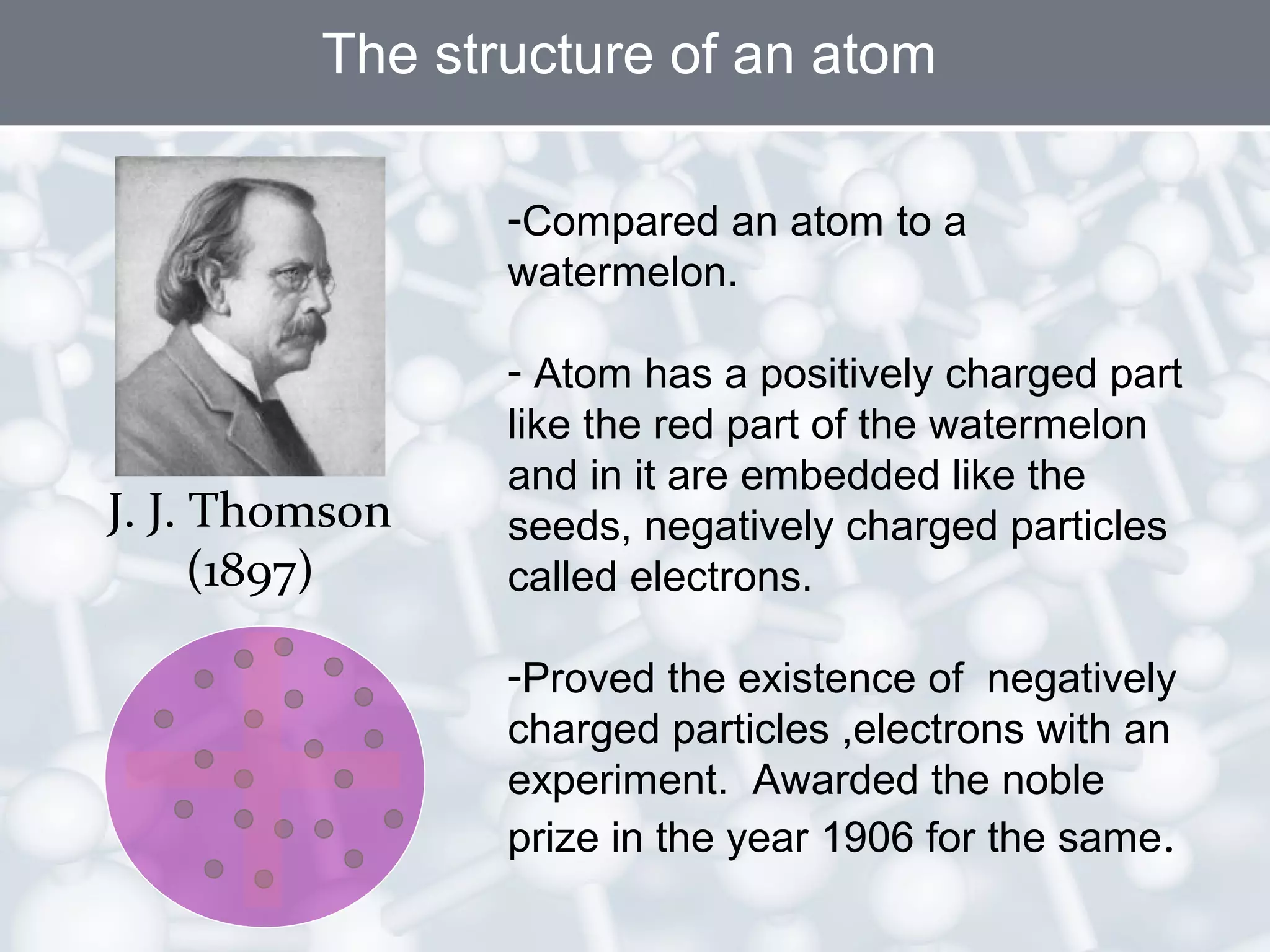
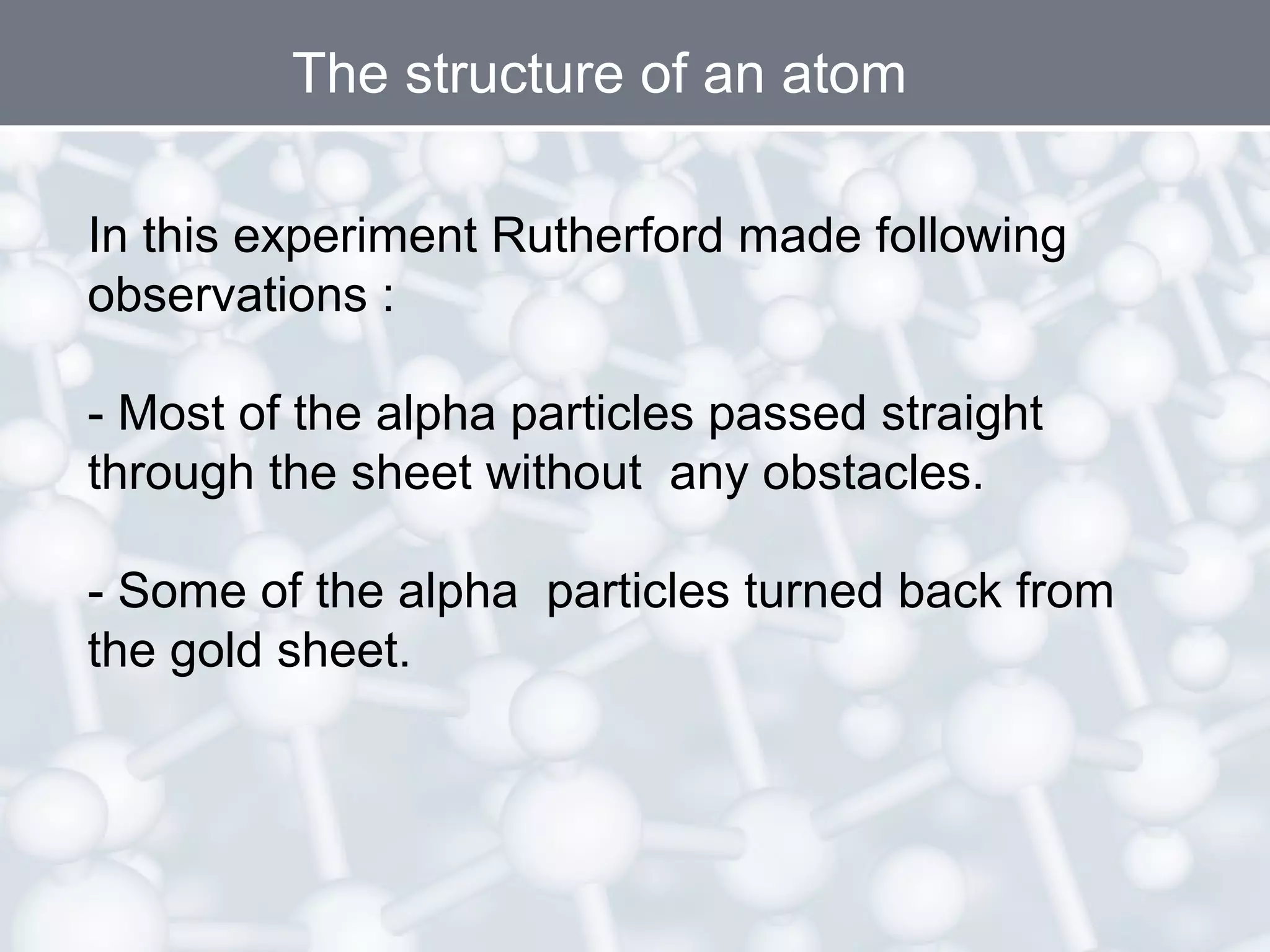
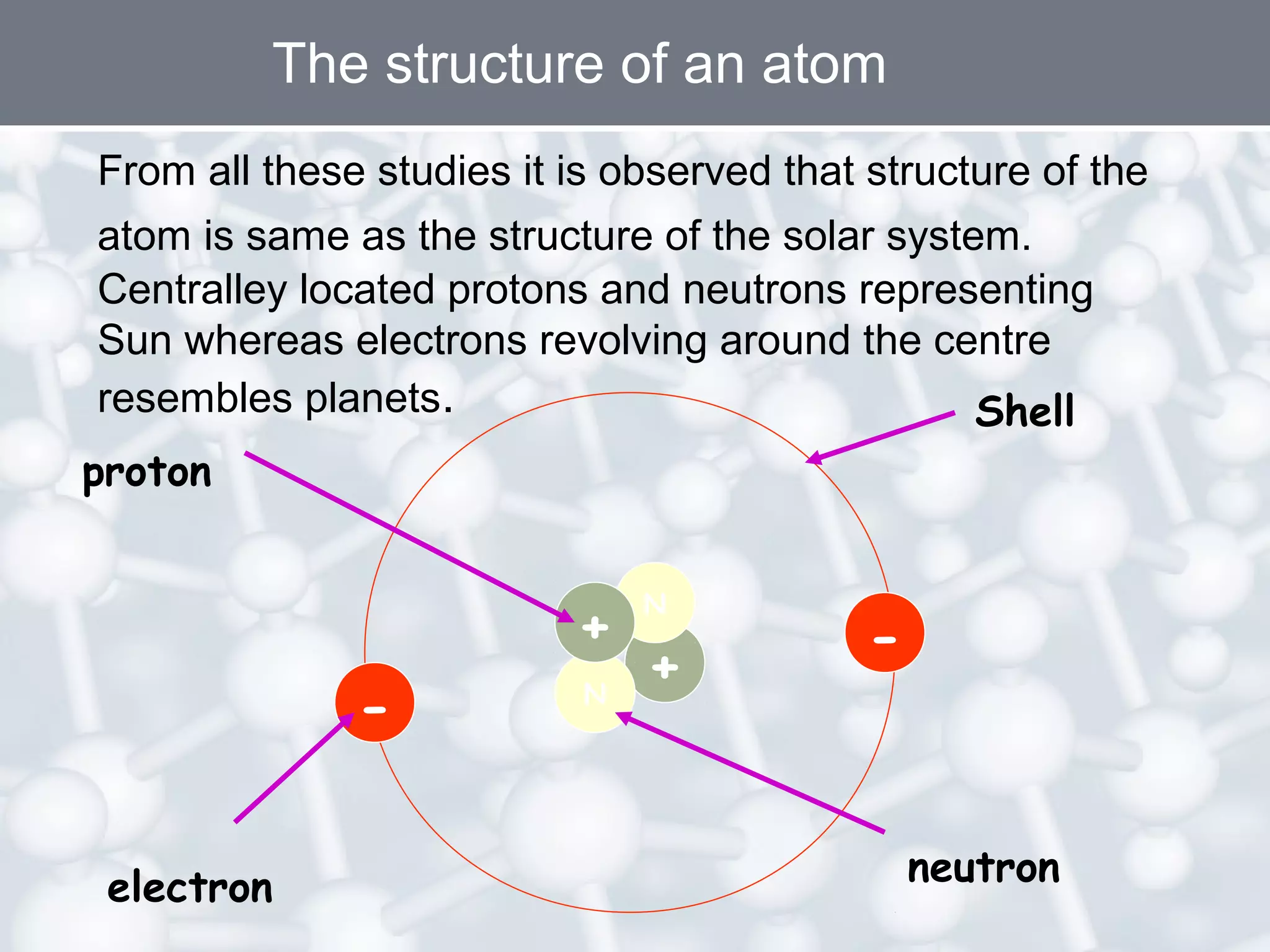
- Atoms are the smallest particles of matter and consist of electrons, protons, and neutrons. Protons and neutrons are located in the nucleus, while electrons orbit the nucleus.
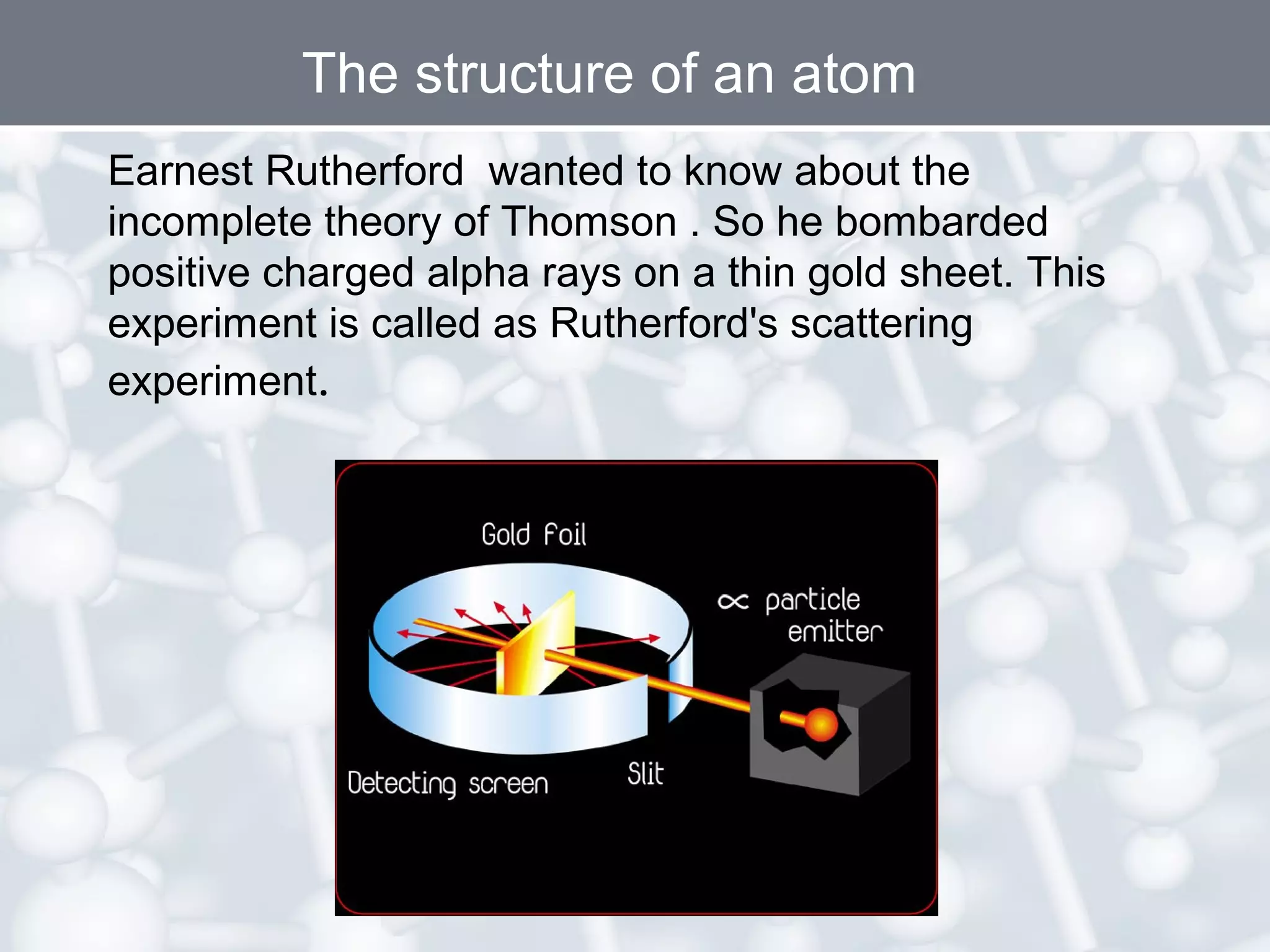
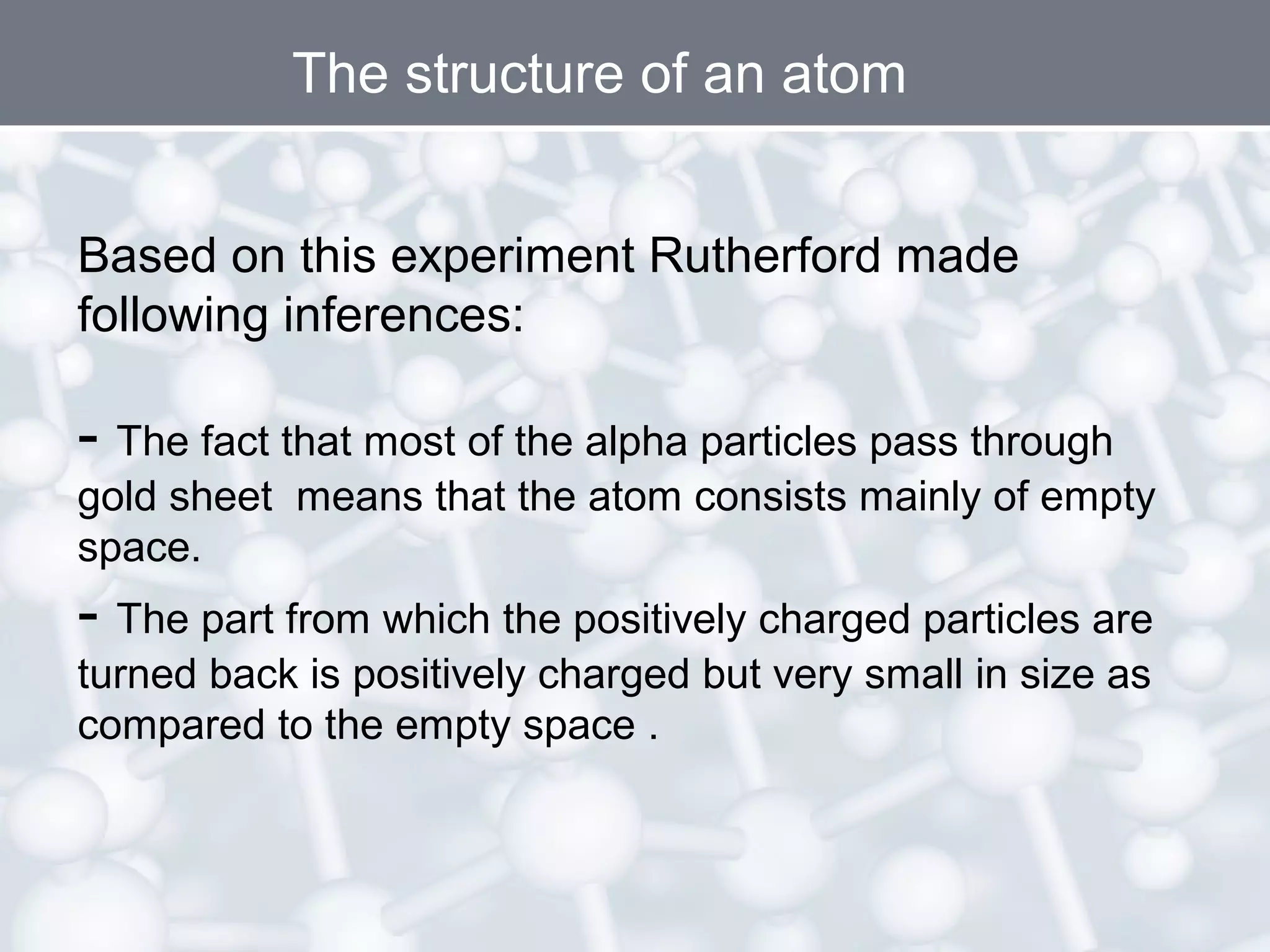
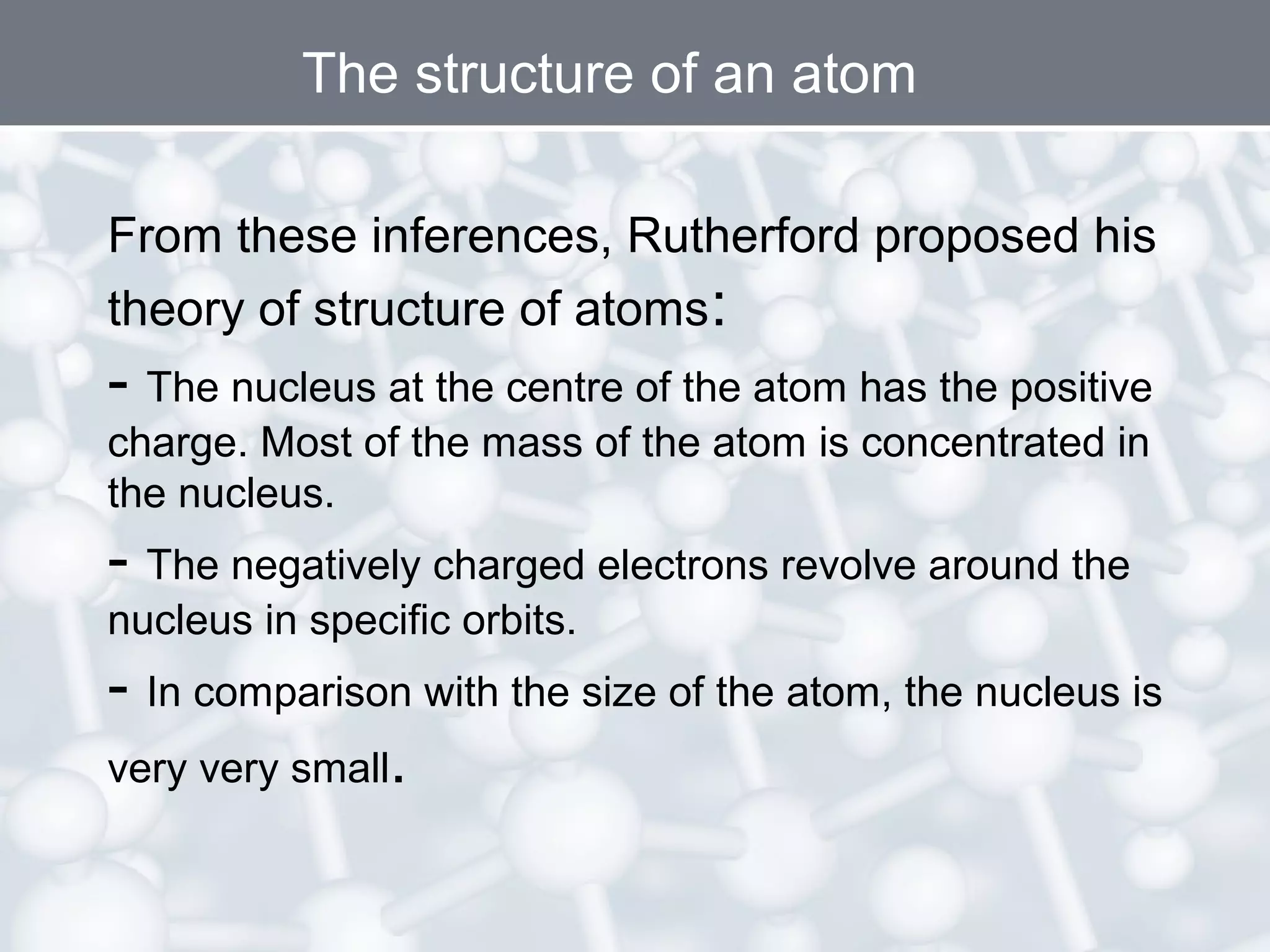
- Rutherford's gold foil experiment showed that atoms are mostly empty space, with a small, dense nucleus at the center containing positive charge. Electrons orbit the nucleus.
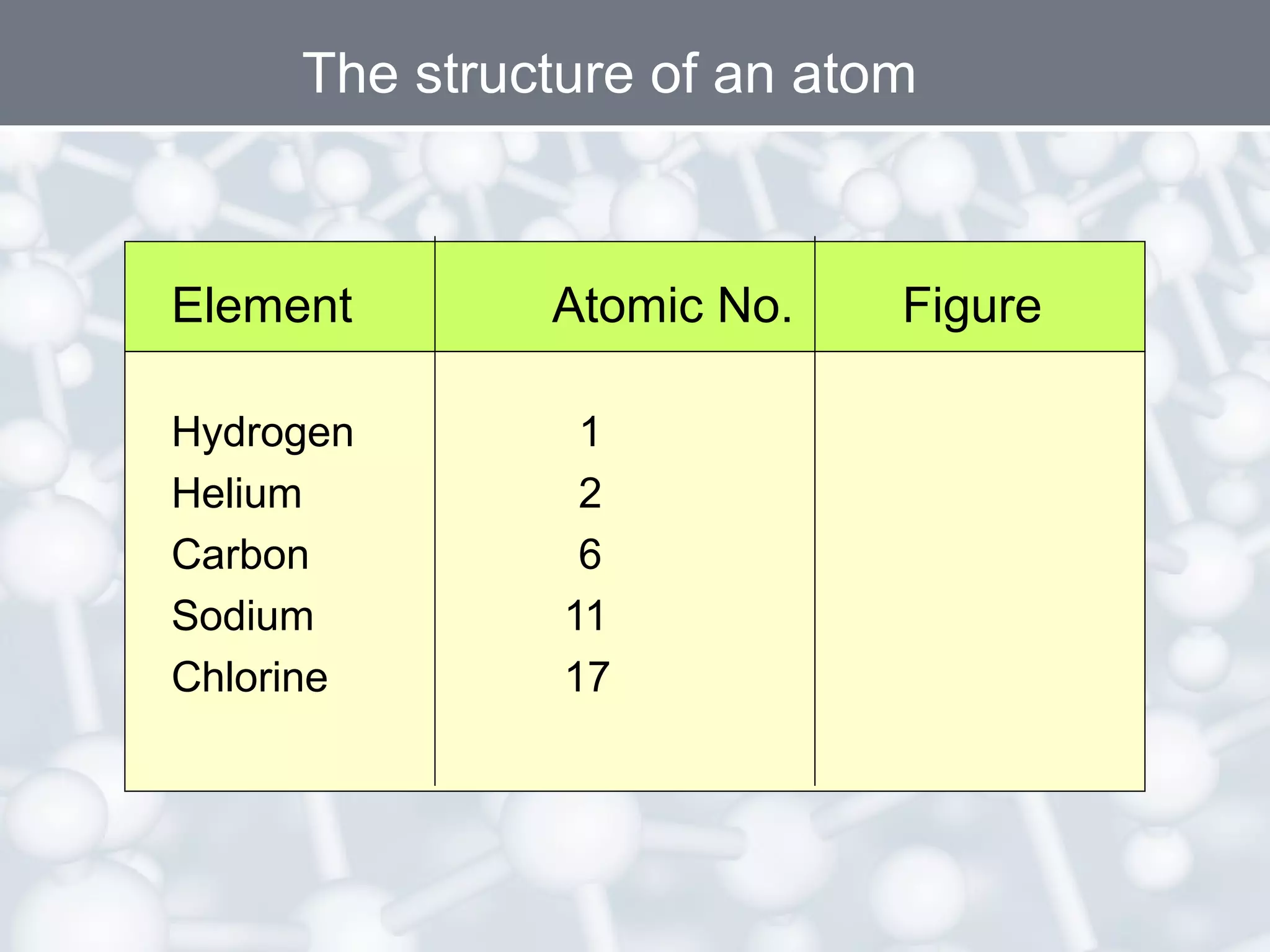
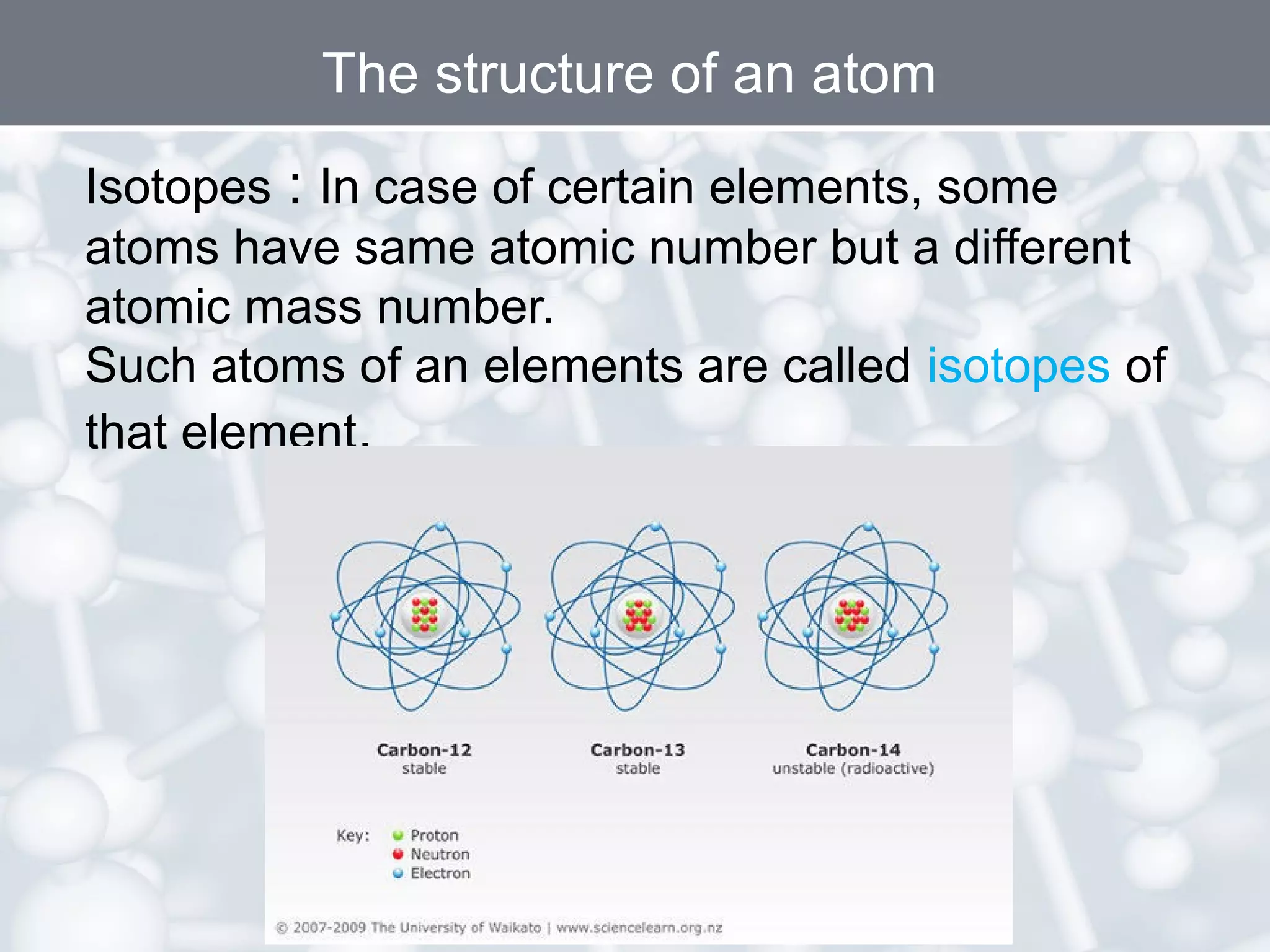
- An atom's atomic number is the number of protons, which determines the element. The mass number is the total number of protons and neutrons. Isotopes are atoms of the same element with different numbers of neutrons.