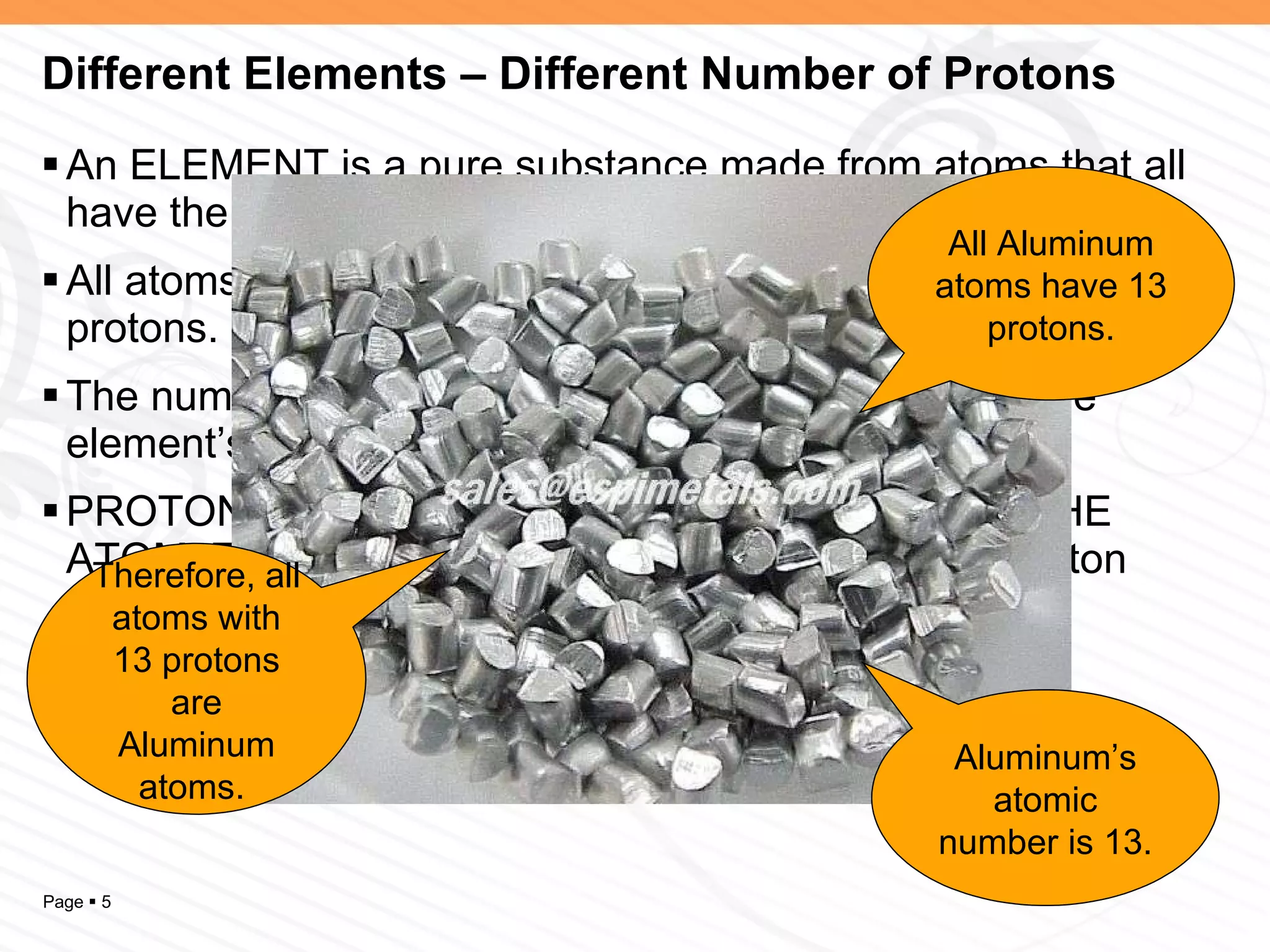
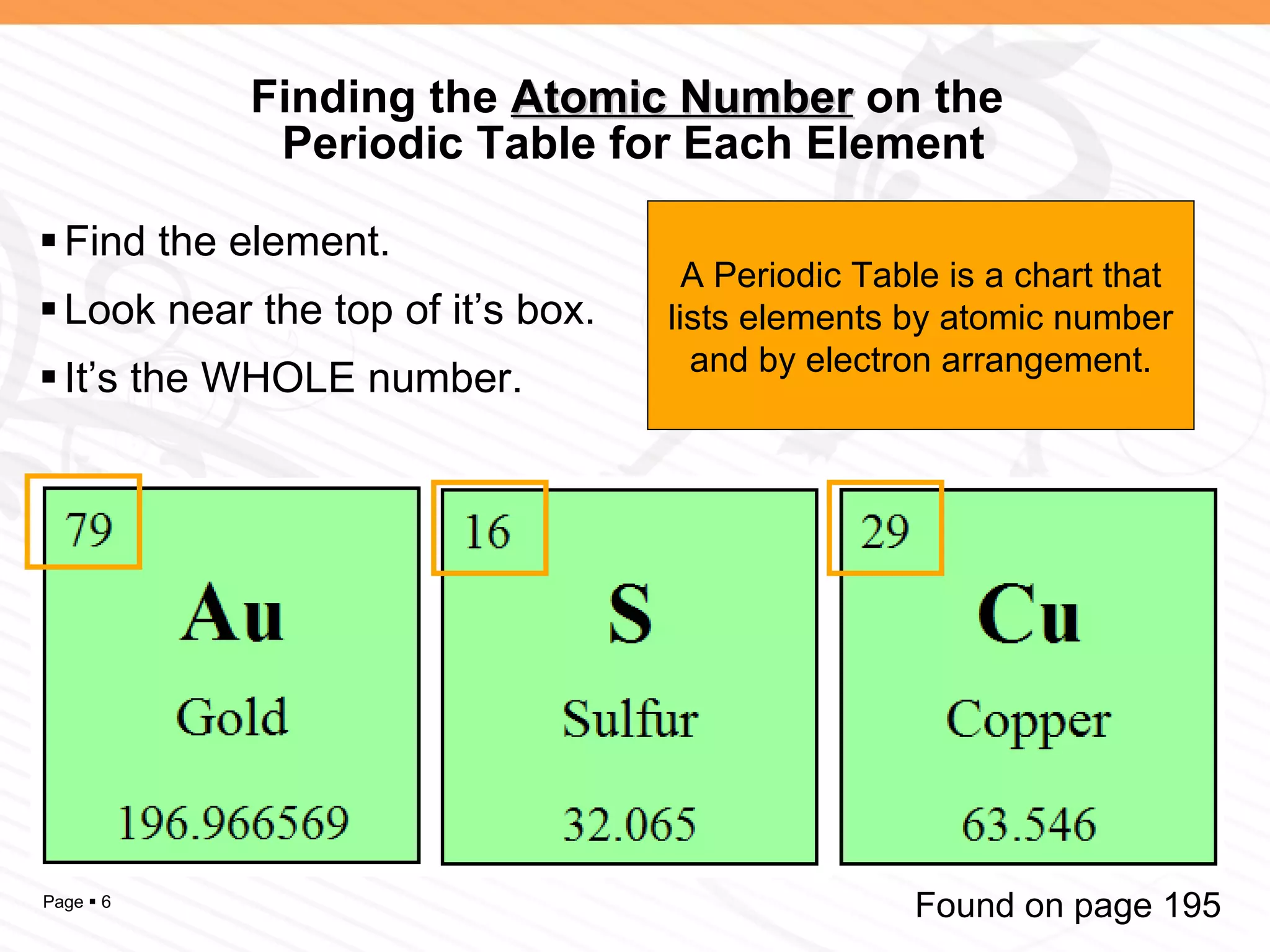
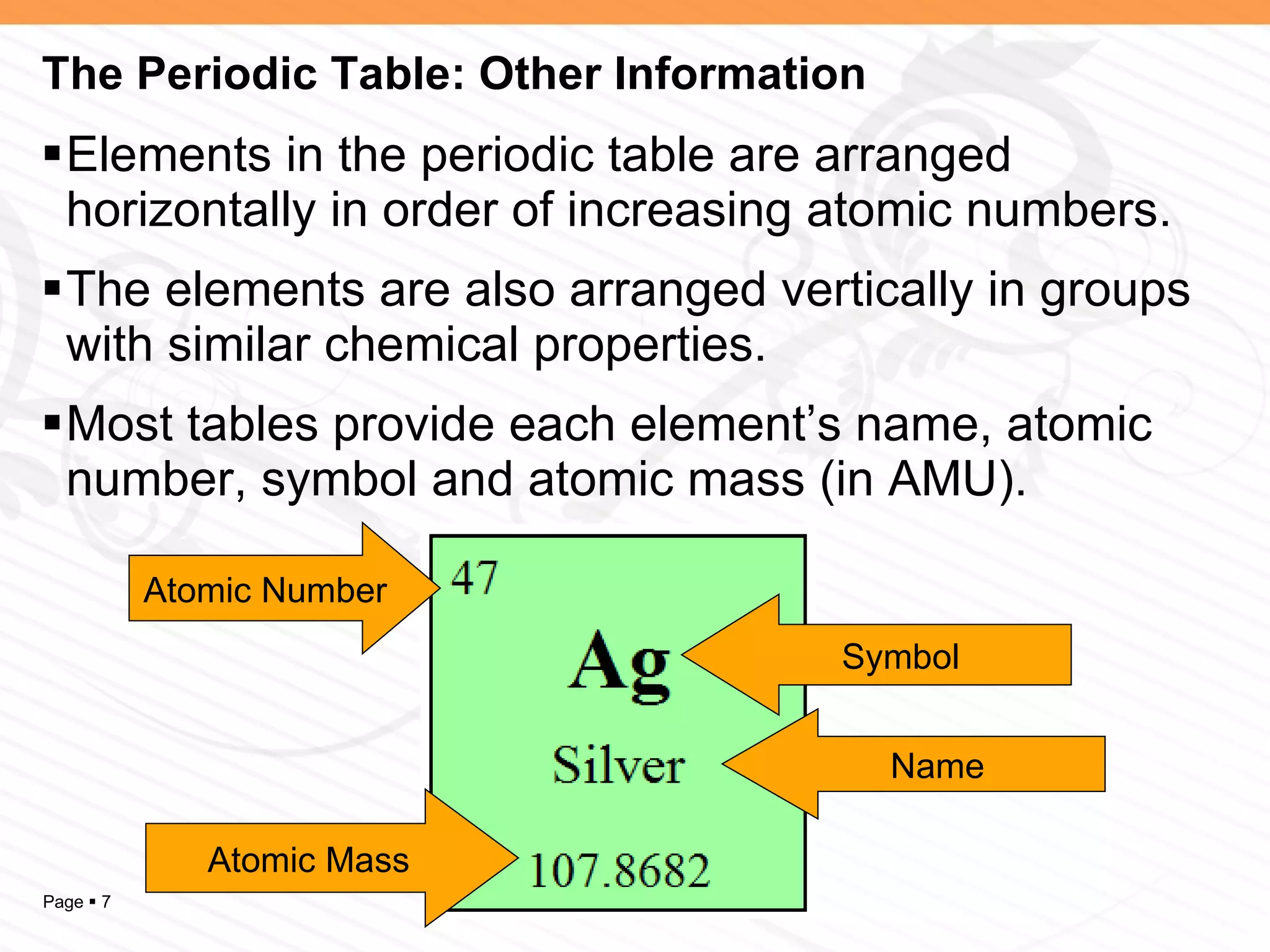
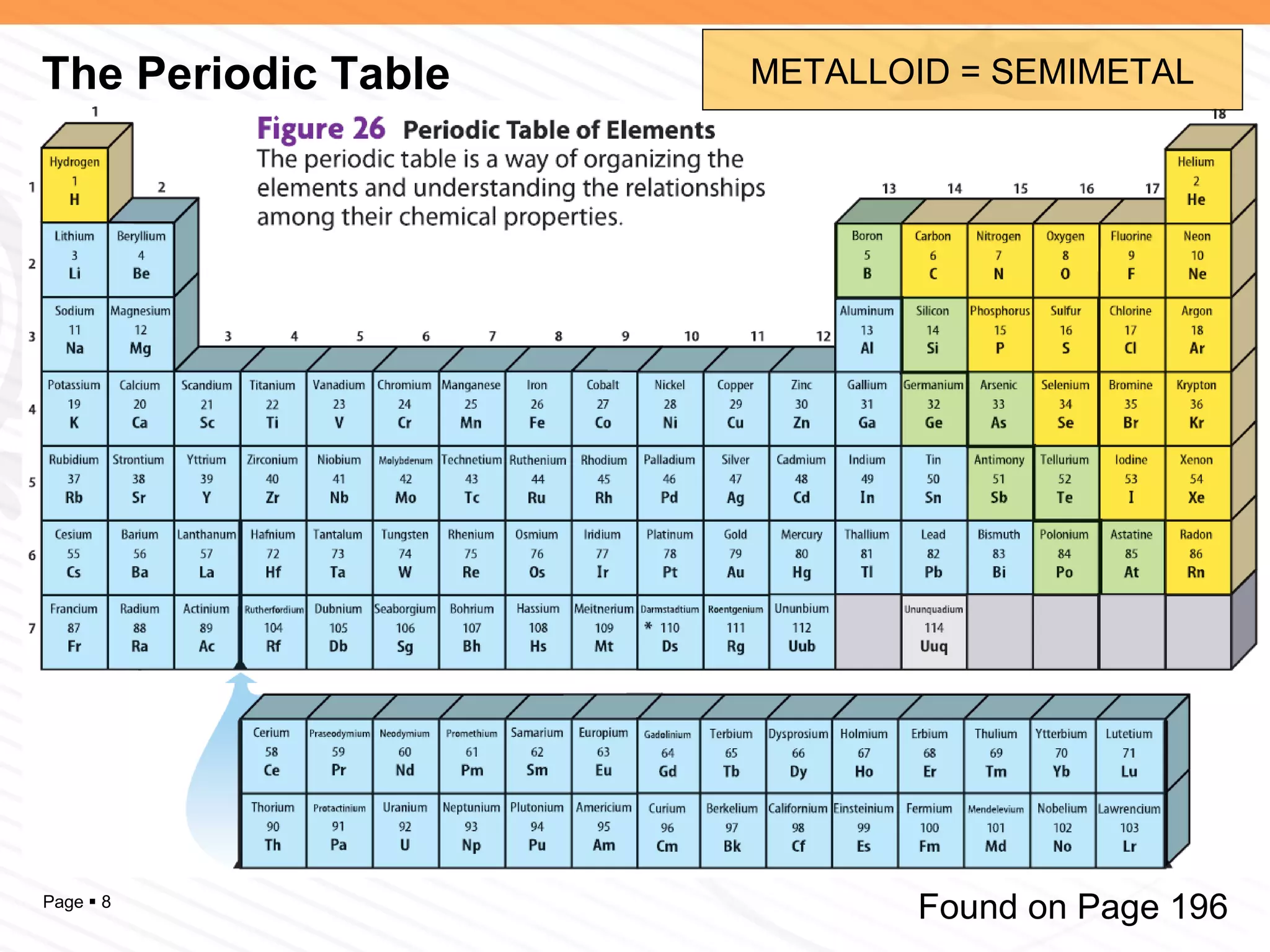
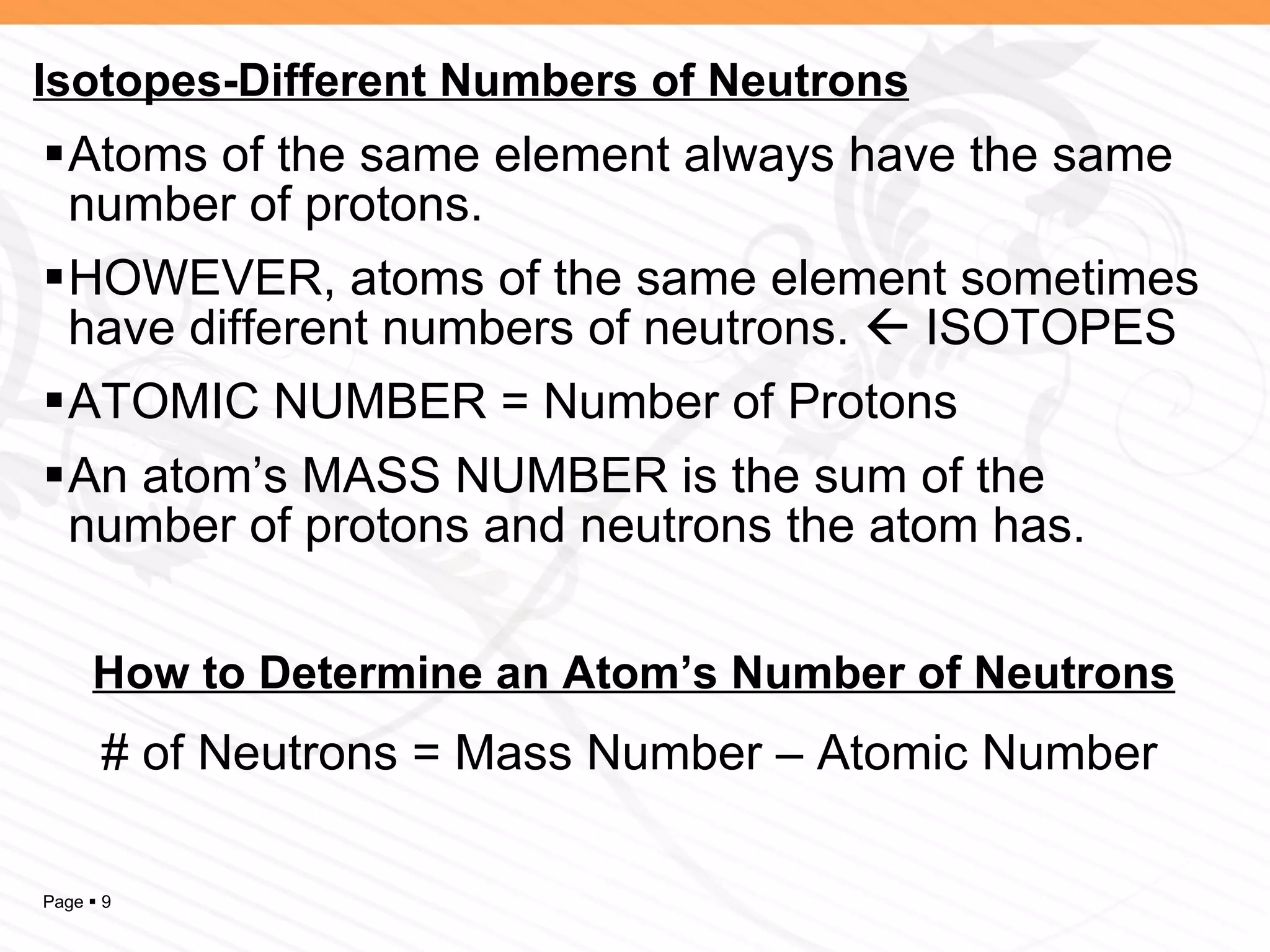
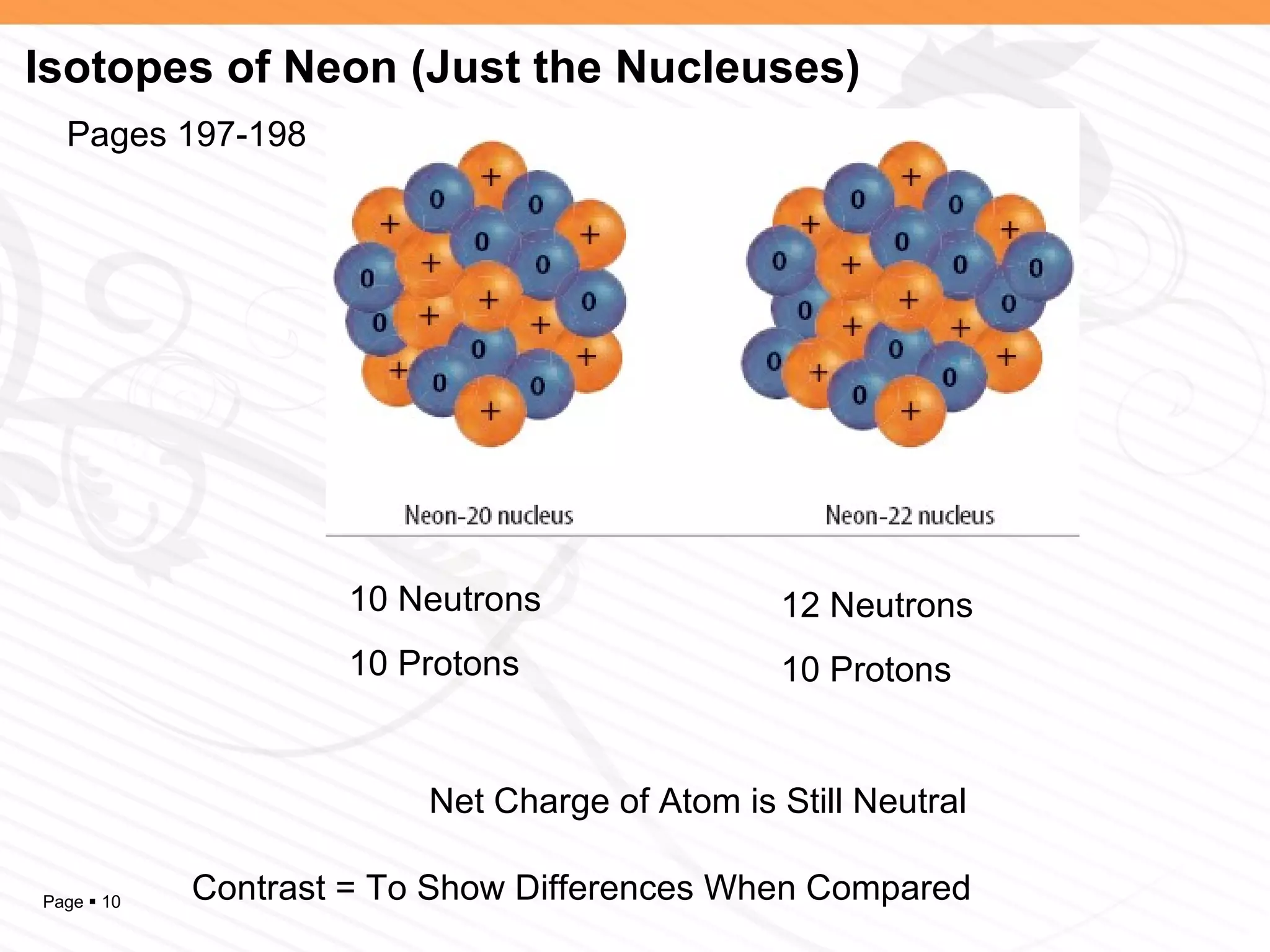
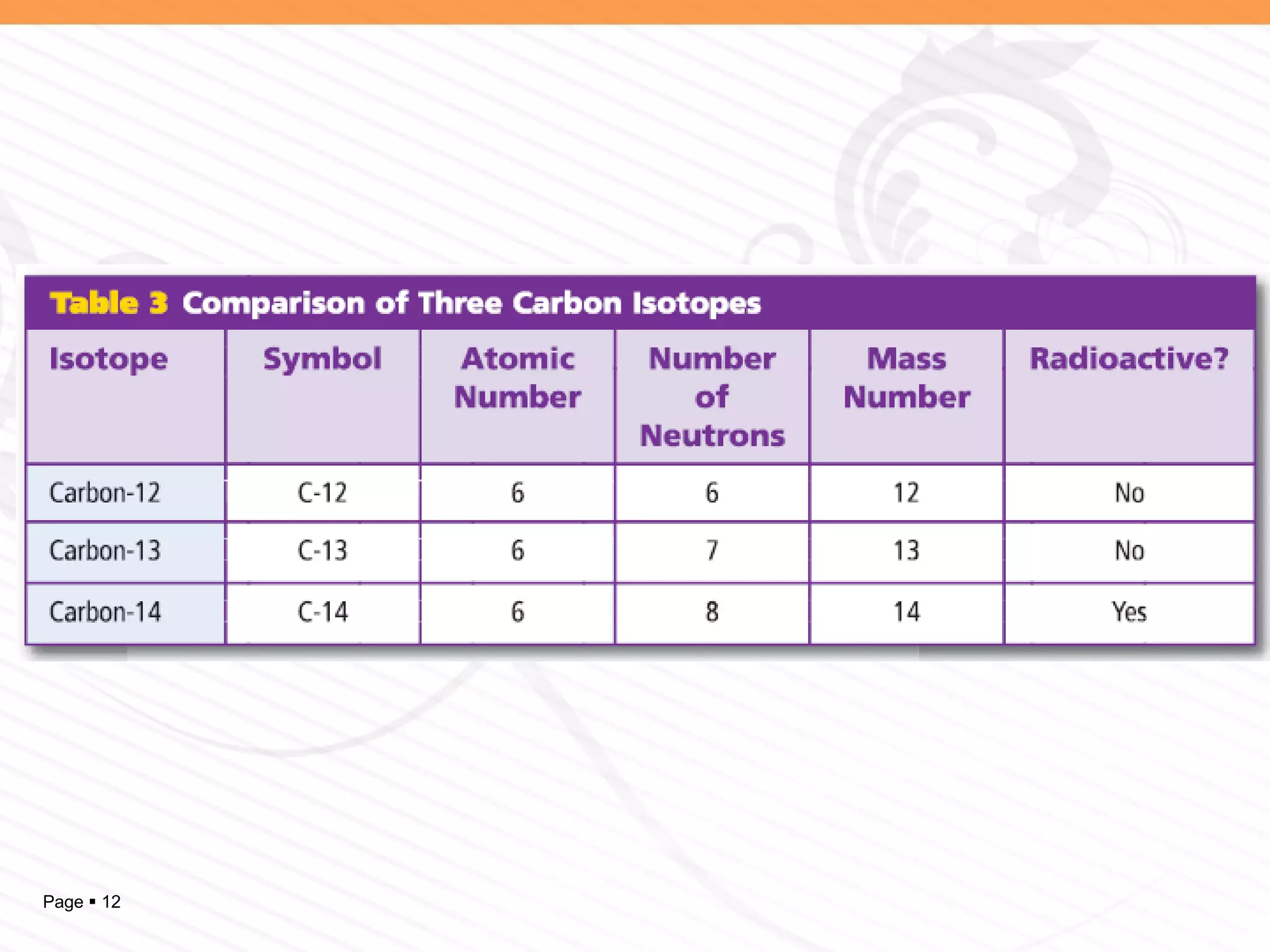
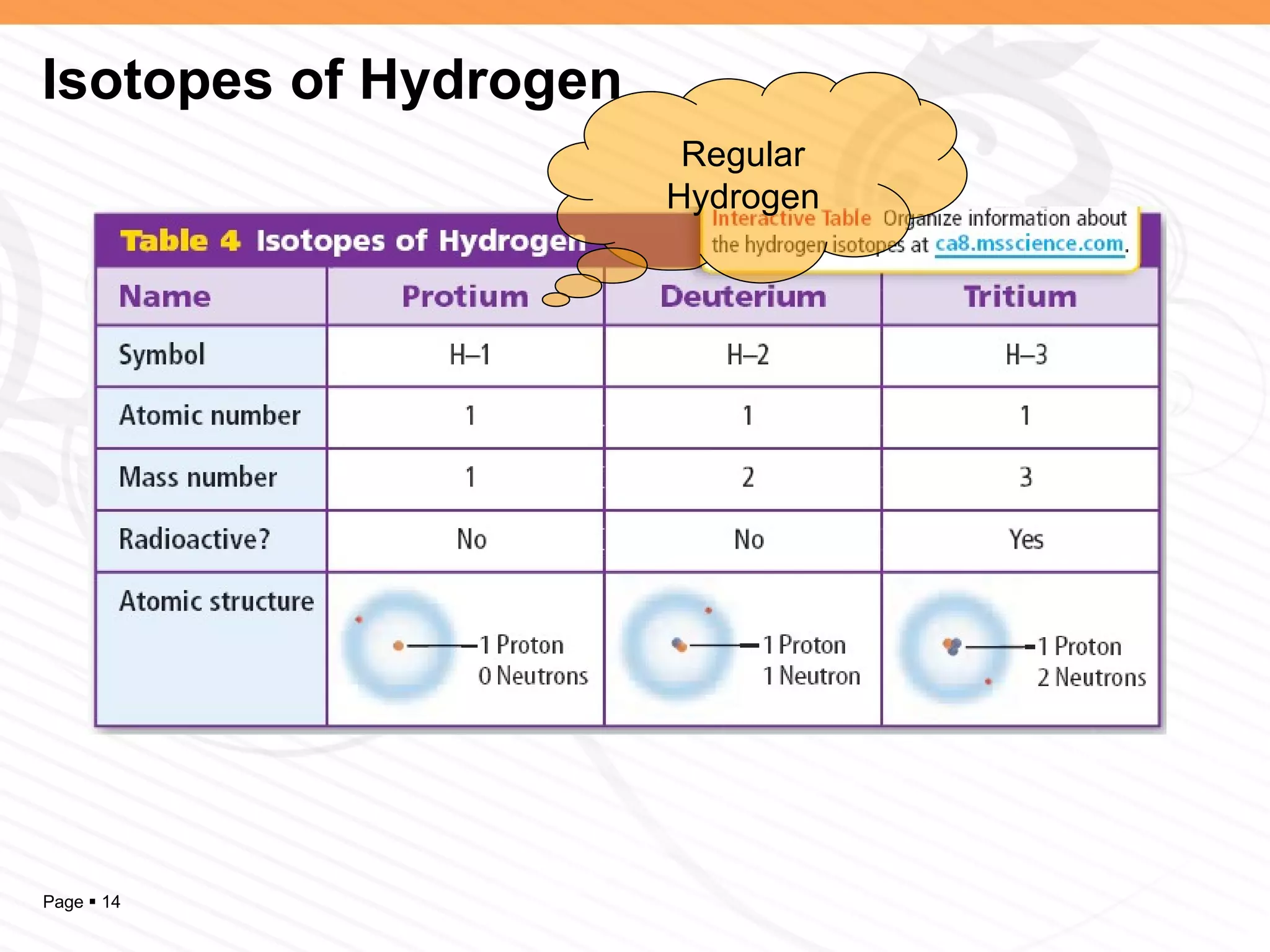
The document discusses the differences between elements, isotopes, and ions at the atomic level. Elements are defined by their atomic number, or number of protons. Isotopes are atoms of the same element that have different numbers of neutrons. Ions are atoms that have gained or lost electrons, giving them a positive or negative charge. The periodic table organizes elements by their atomic number and provides other atomic information. Atoms of the same element can have different isotopes depending on their number of neutrons. Atoms can also form ions by gaining or losing electrons.