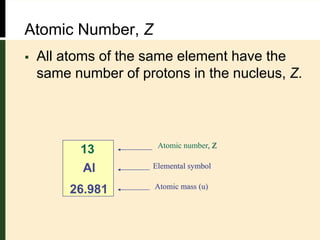
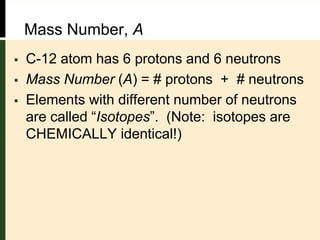
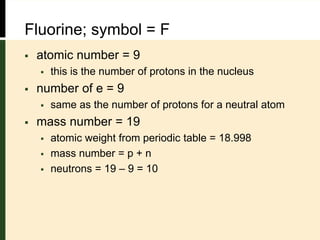
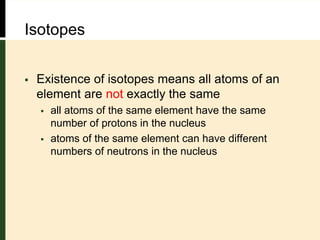
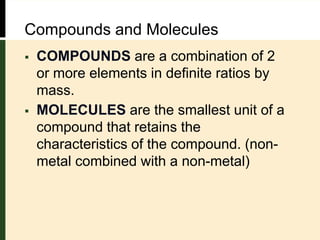
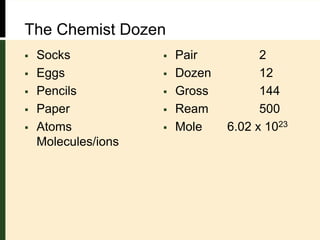
This document provides an overview of atomic structure and bonding. It defines key terms like elements, atoms, subatomic particles, isotopes, ions and molecules. Atoms are made up of protons, neutrons and electrons. The number of protons defines the element and isotopes can have different numbers of neutrons. Electrons determine whether an atom is neutral or an ion. Atoms combine to form compounds and the smallest units of compounds are molecules. The mole is used to quantify very large numbers of small particles like atoms and molecules, with one mole equal to Avogadro's number of 6.022x1023 particles.